miR-155 Modifies Inflammation, Endothelial Activation and Blood-Brain Barrier Dysfunction in Cerebral Malaria
- PMID: 28182191
- PMCID: PMC5364112
- DOI: 10.2119/molmed.2016.00139
miR-155 Modifies Inflammation, Endothelial Activation and Blood-Brain Barrier Dysfunction in Cerebral Malaria
Abstract
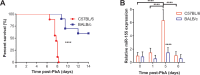
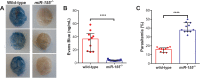
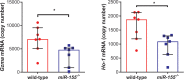
miR-155 has been shown to participate in host response to infection and neuro-inflammation via negative regulation of blood-brain-barrier (BBB) integrity and T cell function. We hypothesized that miR-155 may contribute to the pathogenesis of cerebral malaria (CM). To test this hypothesis, we used a genetic approach to modulate miR-155 expression in an experimental model of cerebral malaria (ECM). In addition, an engineered endothelialized microvessel system and serum samples from Ugandan children with CM were used to examine an anti-miR-155 as a potential adjunctive therapeutic for severe malaria. Despite higher parasitemia, survival was significantly improved in miR-155-/- mice vs. wild-type littermate mice in ECM. Improved survival was associated with preservation of BBB integrity and reduced endothelial activation, despite increased levels of pro-inflammatory cytokines. Pre-treatment with antagomir-155 reduced vascular leak induced by human CM sera in an ex vivo endothelial microvessel model. These data provide evidence supporting a mechanistic role for miR-155 in host response to malaria via regulation of endothelial activation, microvascular leak and BBB dysfunction in CM.
Keywords: antagomir; cerebral malaria; microRNAs; microvessels; plasmodium.
Conflict of interest statement
The authors declare they have no competing interests as defined by
Figures




References
-
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. - PMC - PubMed
-
- Dondorp A, Nosten F, Stepniewska K, Day N, White N. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2010;366:717–25. - PubMed
-
- Kim H, Higgins S, Liles WC, Kain KC. Endothelial activation and dysregulation in malaria: a potential target for novel therapeutics. Curr. Opin. Hematol. 2011;18:177–85. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

