Uncovering sensory axonal dysfunction in asymptomatic type 2 diabetic neuropathy
- PMID: 28182728
- PMCID: PMC5300160
- DOI: 10.1371/journal.pone.0171223
Uncovering sensory axonal dysfunction in asymptomatic type 2 diabetic neuropathy
Abstract
This study investigated sensory and motor nerve excitability properties to elucidate the development of diabetic neuropathy. A total of 109 type 2 diabetes patients were recruited, and 106 were analyzed. According to neuropathy severity, patients were categorized into G0, G1, and G2+3 groups using the total neuropathy score-reduced (TNSr). Patients in the G0 group were asymptomatic and had a TNSr score of 0. Sensory and motor nerve excitability data from diabetic patients were compared with data from 33 healthy controls. Clinical assessment, nerve conduction studies, and sensory and motor nerve excitability testing data were analyzed to determine axonal dysfunction in diabetic neuropathy. In the G0 group, sensory excitability testing revealed increased stimulus for the 50% sensory nerve action potential (P<0.05), shortened strength-duration time constant (P<0.01), increased superexcitability (P<0.01), decreased subexcitability (P<0.05), decreased accommodation to depolarizing current (P<0.01), and a trend of decreased accommodation to hyperpolarizing current in threshold electrotonus. All the changes progressed into G1 (TNSr 1-8) and G2+3 (TNSr 9-24) groups. In contrast, motor excitability only had significantly increased stimulus for the 50% compound motor nerve action potential (P<0.01) in the G0 group. This study revealed that the development of axonal dysfunction in sensory axons occurred prior to and in a different fashion from motor axons. Additionally, sensory nerve excitability tests can detect axonal dysfunction even in asymptomatic patients. These insights further our understanding of diabetic neuropathy and enable the early detection of sensory axonal abnormalities, which may provide a basis for neuroprotective therapeutic approaches.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



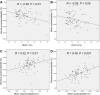
References
-
- Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31–40. Epub 2011/06/28. 10.1016/S0140-6736(11)60679-X - DOI - PubMed
-
- Martin CL, Albers JW, Pop-Busui R. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):31–8. Epub 2013/12/21. PubMed Central PMCID: PMCPmc3868000. 10.2337/dc13-2114 - DOI - PMC - PubMed
-
- American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38 Suppl 1:S1–93. Epub 2014/12/30.
-
- Van Acker K, Bouhassira D, De Bacquer D, Weiss S, Matthys K, Raemen H, et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab. 2009;35(3):206–13. Epub 2009/03/20. 10.1016/j.diabet.2008.11.004 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

