Third generation drug eluting stent (DES) with biodegradable polymer in diabetic patients: 5 years follow-up
- PMID: 28183306
- PMCID: PMC5301341
- DOI: 10.1186/s12933-017-0500-3
Third generation drug eluting stent (DES) with biodegradable polymer in diabetic patients: 5 years follow-up
Erratum in
-
Erratum to: Third generation drug eluting stent (DES) with biodegradable polymer in diabetic patients: 5 years follow-up.Cardiovasc Diabetol. 2017 May 4;16(1):59. doi: 10.1186/s12933-017-0541-7. Cardiovasc Diabetol. 2017. PMID: 28472961 Free PMC article. No abstract available.
Abstract
Objective: To report the long-term safety and efficacy data of a third generation drug eluting stent (DES) with biodegradable polymer in the complex patient population of diabetes mellitus after a follow-up period of 5 years.
Background: After percutaneous coronary intervention patients with diabetes mellitus are under higher risk of death, restenosis and stent thrombosis (ST) compared to non-diabetic patients.
Methods: In 126 centers worldwide 3067 patients were enrolled in the NOBORI 2 registry, 888 patients suffered from diabetes mellitus (DM), 213 of them (14%) being insulin dependent (IDDM). Five years follow-up has been completed in this study.
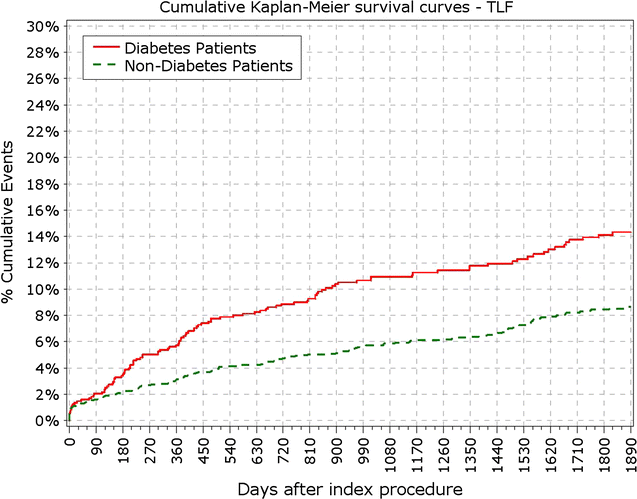
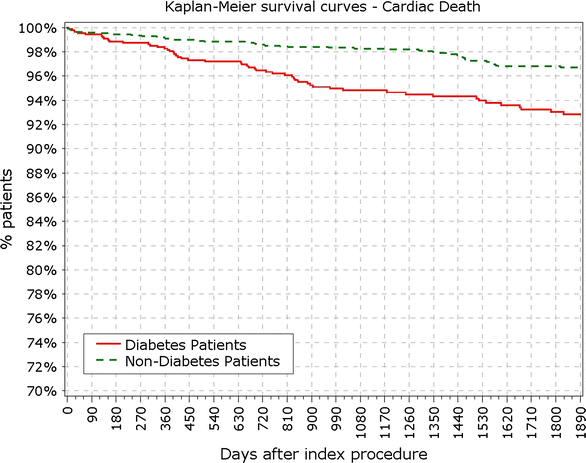
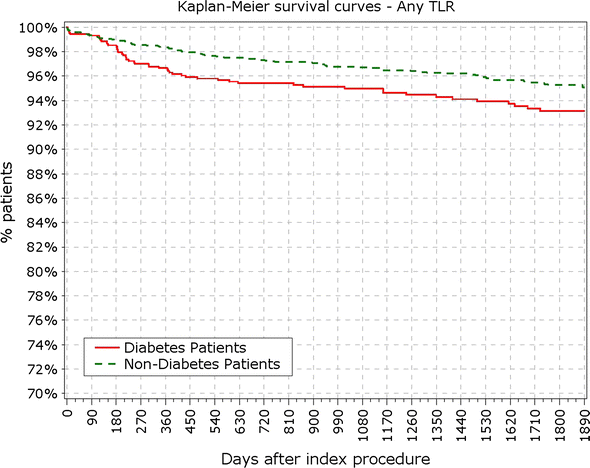
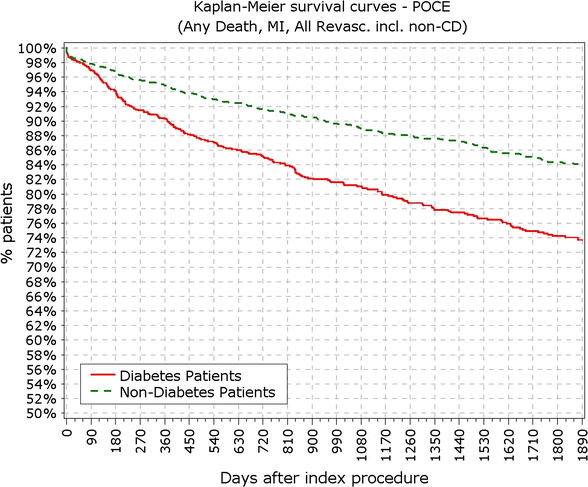
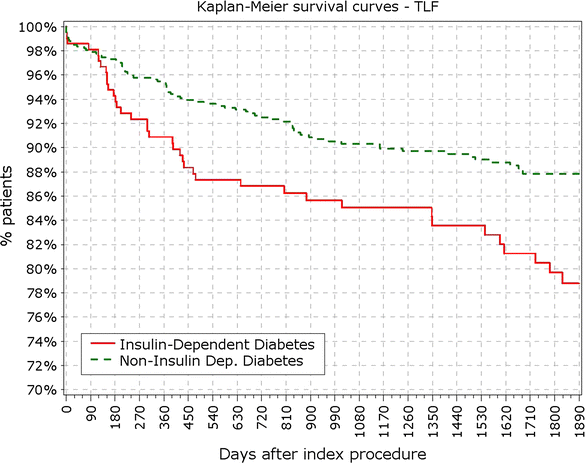
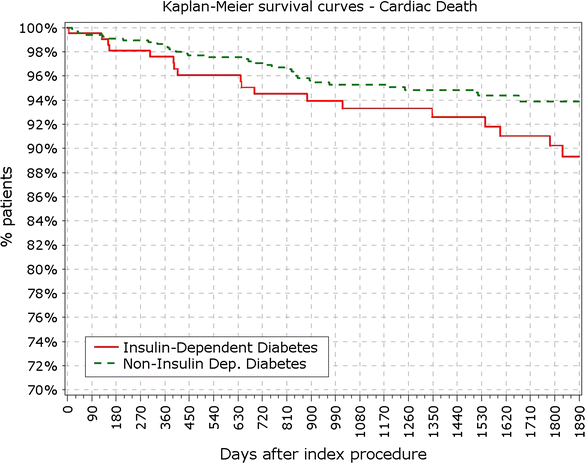
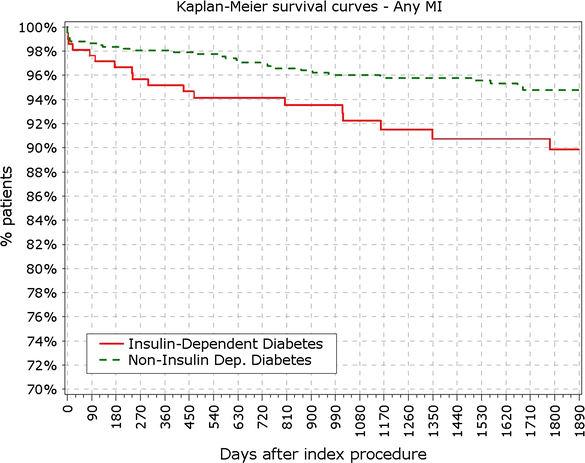
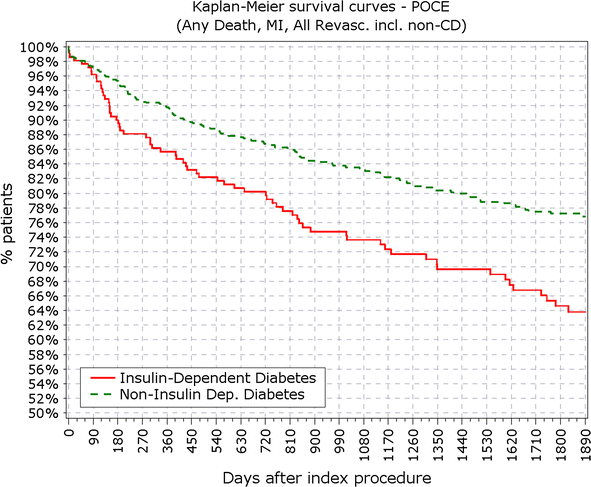
Results: At 5 years, 89.3% of the patients were available for follow-up. The reported target lesion failure (TLF) rates at 5 years were 12.39% in DM group and 7.34% in non-DM group; (p < 0.0001). In the DM group, the TLF rate in patients with IDDM was significantly higher than in the non-IDDM subgroup (17.84 vs. 10.67%; p < 0.01). The rate of ST at 5 years was not different among diabetic versus non-diabetic patients or IDDM versus NIDDM. Only 10 (<0.4%) very late stent thrombotic events beyond 12 months occurred.
Conclusions: The Nobori DES performed well in patients with DM. As expected patients with DM, particularly those with IDDM, had worse outcomes. However, the very low rate of very late stent thrombosis in IDDM patients might have significant clinical value in the treatment of these patients. Clinical trial registration ISRCTN81649913; http://www.controlled-trials.com/isrctn/search.html?srch=81649913&sort=3&dir=desc&max=10.
Figures
References
-
- Sousa JE, Costa MA, Abizaid A, Abizaid AS, Feres F, Pinto IM, Seixas AC, Staico R, Mattos LA, Sousa AG, et al. Lack of neointimal proliferation after implantation of sirolimus-coated stents in human coronary arteries: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2001;103(2):192–195. doi: 10.1161/01.CIR.103.2.192. - DOI - PubMed
-
- Finn AV, Kolodgie FD, Harnek J, Guerrero LJ, Acampado E, Tefera K, Skorija K, Weber DK, Gold HK, Virmani R. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation. 2005;112(2):270–278. doi: 10.1161/CIRCULATIONAHA.104.508937. - DOI - PubMed
-
- Sarno G, Lagerqvist B, Nilsson J, Frobert O, Hambraeus K, Varenhorst C, Jensen UJ, Todt T, Gotberg M, James SK. Stent thrombosis in new-generation drug-eluting stents in patients with STEMI undergoing primary PCI: a report from SCAAR. J Am Coll Cardiol. 2014;64(1):16–24. doi: 10.1016/j.jacc.2014.04.022. - DOI - PubMed
-
- Chevalier B, Silber S, Park SJ, Garcia E, Schuler G, Suryapranata H, Koolen J, Hauptmann KE, Wijns W, Morice MC, et al. Randomized comparison of the Nobori Biolimus A9-eluting coronary stent with the Taxus Liberte paclitaxel-eluting coronary stent in patients with stenosis in native coronary arteries: the NOBORI 1 trial—Phase 2. Circ Cardiovasc Interv. 2009;2(3):188–195. doi: 10.1161/CIRCINTERVENTIONS.108.823443. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

