Biomarker analysis of the NeoSphere study: pertuzumab, trastuzumab, and docetaxel versus trastuzumab plus docetaxel, pertuzumab plus trastuzumab, or pertuzumab plus docetaxel for the neoadjuvant treatment of HER2-positive breast cancer
- PMID: 28183321
- PMCID: PMC5299741
- DOI: 10.1186/s13058-017-0806-9
Biomarker analysis of the NeoSphere study: pertuzumab, trastuzumab, and docetaxel versus trastuzumab plus docetaxel, pertuzumab plus trastuzumab, or pertuzumab plus docetaxel for the neoadjuvant treatment of HER2-positive breast cancer
Abstract
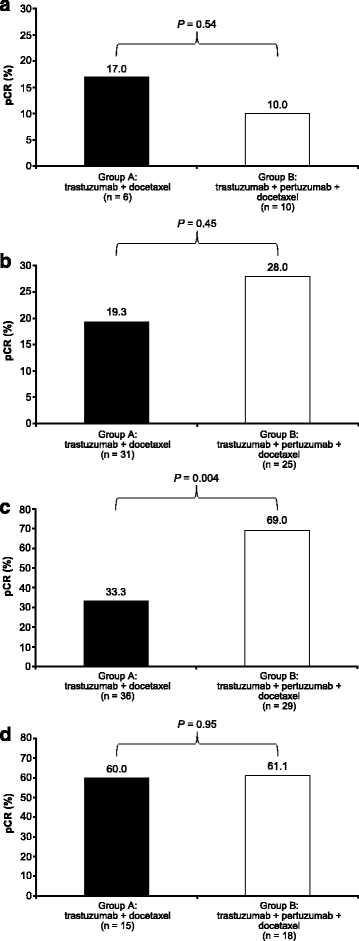
Background: NeoSphere showed significantly higher pathologic complete response (pCR) with neoadjuvant pertuzumab, trastuzumab, and docetaxel compared with trastuzumab plus docetaxel, pertuzumab plus trastuzumab, or pertuzumab plus docetaxel. We assessed associations between human epidermal growth factor receptor 2 (HER2) pathway-related biomarkers and clinical outcome in response to these regimens.
Methods: Tumor, serum, and whole blood samples were collected at baseline and post neoadjuvant treatment before surgery. Associations between biomarkers and pCR, and between biomarkers and clinical variables were assessed in the overall and estrogen receptor (ER)-positive and ER-negative populations. Changes in serum marker levels between baseline and post-neoadjuvant treatment were examined.
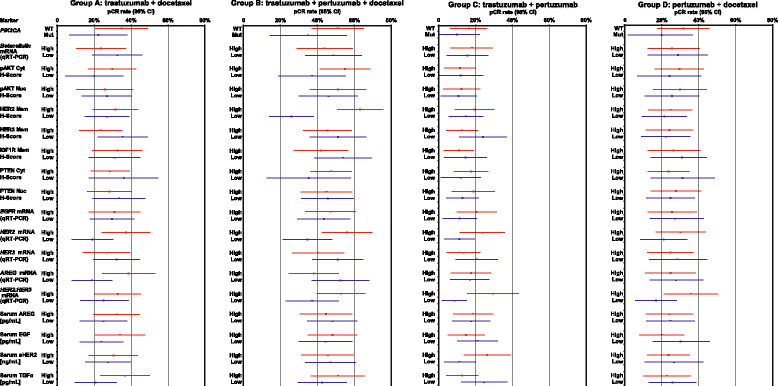
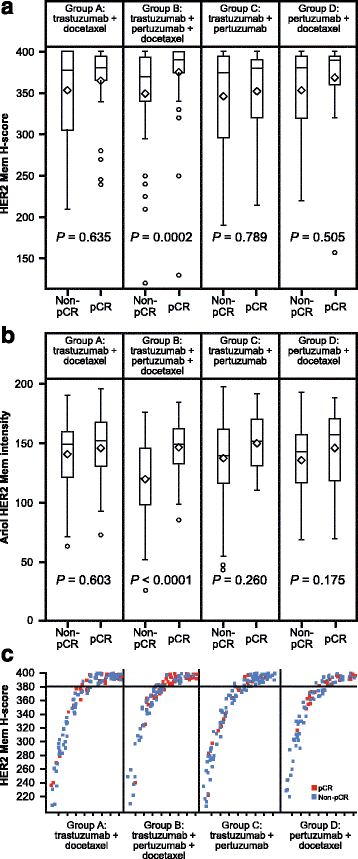
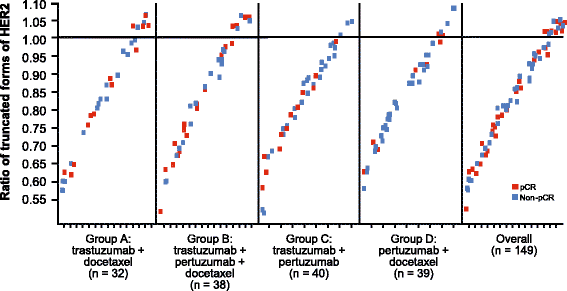
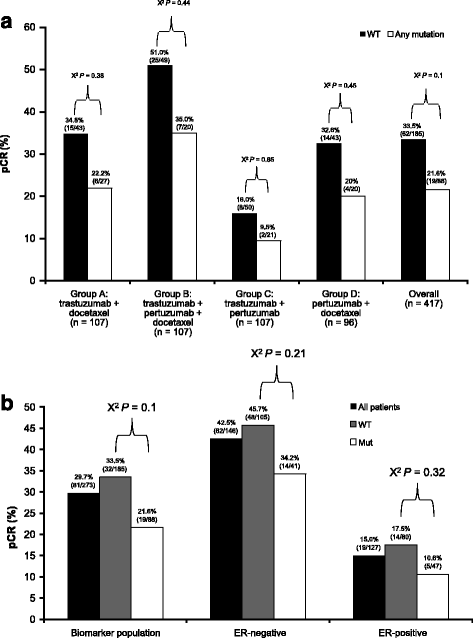
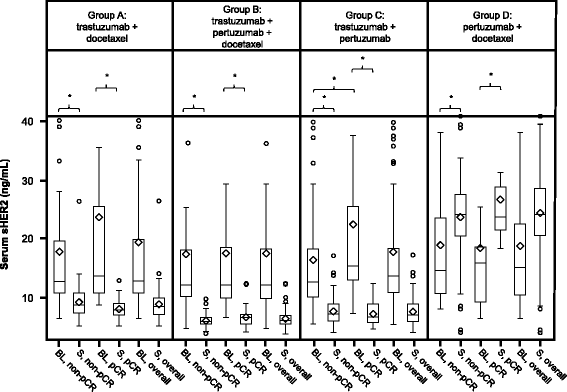
Results: No markers were associated with pCR across all groups; however, significant associations were observed for two markers in individual groups. High HER2 was significantly associated with higher pCR rates (P = 0.001) and a significant treatment interaction (P = 0.0236) with pertuzumab, trastuzumab, and docetaxel (odds ratio 2.07, P = 0.01). Low serum transforming growth factor alpha (TGFα) was associated with higher pCR rates with pertuzumab plus trastuzumab (P = 0.04) without a significant treatment interaction. Presence of truncated HER2 did not affect pCR. A non-significant decreased pCR benefit was observed consistently across groups in patients with mutated PIK3CA while the treatment benefit from pertuzumab was maintained when comparing the trastuzumab plus docetaxel and pertuzumab, trastuzumab, and docetaxel groups. Notably, PIK3CA exon 9 mutations were associated with residual disease (pooled groups), which was not found for exon 20 mutations. Serum HER2 extracellular domain levels were significantly increased between baseline and post-neoadjuvant treatment in the non-trastuzumab-treated group, and decreased in the trastuzumab-containing groups (likely due to trastuzumab's mechanism of action). Differences in biomarker profiles according to ER status were observed.
Conclusions: The observed associations of HER2 protein levels with sensitivity to pertuzumab, and of PIK3CA exon 9 mutation to lack of sensitivity to HER2-targeted monoclonal antibody treatment, warrant further investigation. Previously reported findings of truncated forms of HER2 as resistance markers to HER2-targeted treatment could not be confirmed in NeoSphere. Conventional HER2 assessment should continue and HER2 remains the only biomarker suitable for patient selection in this population.
Trial registration: Clinicaltrials.gov, NCT00545688 . Registered on 16 October 2007.
Keywords: Biomarker; Breast cancer; Docetaxel; HER2; Neoadjuvant; Pertuzumab; Trastuzumab.
Figures
References
-
- Barok M, Isola J, Pályi-Krekk Z, Nagy P, Juhász I, Vereb G, Kauraniemi P, Kapanen A, Tanner M, Vereb G, Szöllösi J. Trastuzumab causes antibody-dependent cellular cytotoxicity-mediated growth inhibition of submacroscopic JIMT-1 breast cancer xenografts despite intrinsic drug resistance. Mol Cancer Ther. 2007;6:2065–72. doi: 10.1158/1535-7163.MCT-06-0766. - DOI - PubMed
-
- Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (Herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

