Impact of a pharmacist-led medication review on hospital readmission in a pediatric and elderly population: study protocol for a randomized open-label controlled trial
- PMID: 28183322
- PMCID: PMC5301437
- DOI: 10.1186/s13063-017-1798-6
Impact of a pharmacist-led medication review on hospital readmission in a pediatric and elderly population: study protocol for a randomized open-label controlled trial
Abstract
Background: Early hospital readmission of patients after discharge is a public health problem. One major cause of hospital readmission is dysfunctions in integrated pathways between community and hospital care that can cause adverse drug events. Furthermore, the French ENEIS 2 study showed that 1.3% of hospital stays originated from serious adverse drug events in 2009. Pharmacy-led medication reviews at hospital transitions are an effective means of decreasing medication discrepancies when conducted at admission or discharge. However, it is difficult to assess the true impact of pharmacist-led medication reviews in specific high-risk populations, such as pediatric and geriatric populations. In such a context, it is important to demonstrate the effectiveness of medication reconciliation as part of a standardized medication review process-in pediatric and elderly populations-on all-cause readmissions in a large randomized controlled clinical trial. The aim of this study is to assess the impact of the pharmacist-led medication review on the rate of readmissions and/or death after hospital discharge and patient treatment satisfaction.
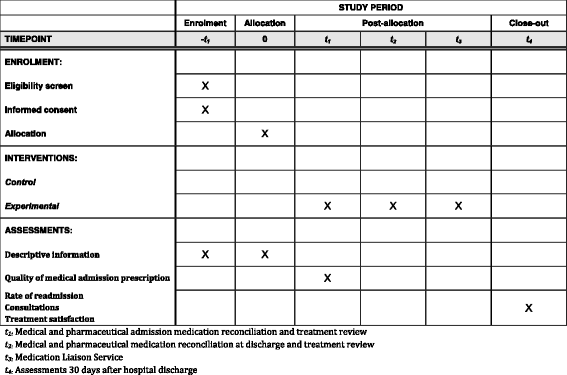
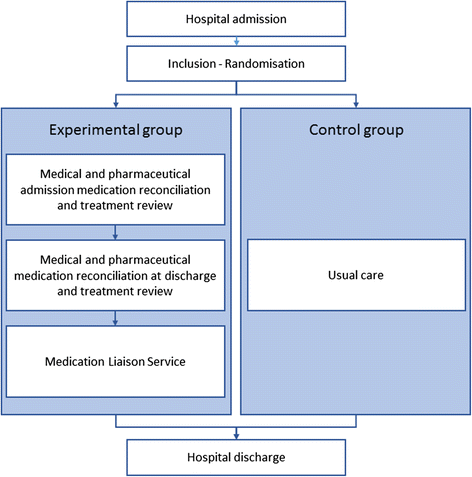
Methods/design: The study is a randomized controlled clinical trial. A total of 1400 hospitalized patients will be randomized in two groups: (1) the experimental group (group receiving a pharmacist-led medication review) and (2) the control group (group receiving usual care). The pharmacist-led medication review process includes medication reconciliation, treatment review and medication liaison service. The primary endpoint will be the rate of readmissions and/or death at 30 days following initial hospitalization discharge. The secondary endpoints will be the rate of hospital readmission, the rate of emergency department visits, the rate of mortality, the number of consultations and patient treatment satisfaction at 30 days following initial hospitalization discharge.
Discussion: A randomized controlled trial provides the most extensive evidence on the impact of pharmacist-led medication reviews on early hospital readmission for extreme age populations.
Trial registration: Current Controlled Trials, NCT02734017 . Registered on 4 May 2016.
Keywords: Clinical pharmacy; Geriatrics; Medication reconciliation; Medication review; Pediatrics; Pharmacists; Randomized controlled trial.
Figures
References
-
- Michel P, others. Enquête nationale sur les événements indésirables liés aux soins (ENEIS). Dir. Rech. Études L’évaluation Stat. DREES. 2011.
-
- Doerper S, Morice S, Piney D, Dony A, Baum T, Perrin F, et al. La conciliation des traitements médicamenteux: logigramme d’une démarche efficiente pour prévenir ou intercepter les erreurs médicamenteuses à l’admission du patient hospitalisé. Pharm Hosp Clin. 2013;48:153–60.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical