Vaccination Targeting Native Receptors to Enhance the Function and Proliferation of Chimeric Antigen Receptor (CAR)-Modified T Cells
- PMID: 28183713
- PMCID: PMC5511585
- DOI: 10.1158/1078-0432.CCR-16-2138
Vaccination Targeting Native Receptors to Enhance the Function and Proliferation of Chimeric Antigen Receptor (CAR)-Modified T Cells
Abstract
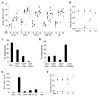
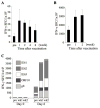
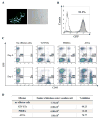
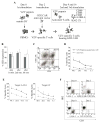
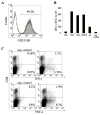
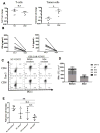
Purpose: The multiple mechanisms used by solid tumors to suppress tumor-specific immune responses are a major barrier to the success of adoptively transferred tumor-specific T cells. As viruses induce potent innate and adaptive immune responses, we hypothesized that the immunogenicity of viruses could be harnessed for the treatment of solid tumors if virus-specific T cells (VST) were modified with tumor-specific chimeric antigen receptors (CAR). We tested this hypothesis using VZV-specific T cells (VZVST) expressing a CAR for GD2, a disialoganglioside expressed on neuroblastoma and certain other tumors, so that the live-attenuated VZV vaccine could be used for in vivo stimulation.Experimental Design: We generated GMP-compliant, GD2.CAR-modified VZVSTs from healthy donors and cancer patients by stimulation of peripheral blood mononuclear cells with overlapping peptide libraries spanning selected VZV antigens, then tested their ability to recognize and kill GD2- and VZV antigen-expressing target cells.Results: Our choice of VZV antigens was validated by the observation that T cells specific for these antigens expanded in vivo after VZV vaccination. VZVSTs secreted cytokines in response to VZV antigens, killed VZV-infected target cells and limited infectious virus spread in autologous fibroblasts. However, while GD2.CAR-modified VZVSTs killed neuroblastoma cell lines on their first encounter, they failed to control tumor cells in subsequent cocultures. Despite this CAR-specific dysfunction, CAR-VZVSTs retained functional specificity for VZV antigens via their TCRs and GD2.CAR function was partially rescued by stimulation through the TCR or exposure to dendritic cell supernatants.Conclusions: Vaccination via the TCR may provide a means to reactivate CAR-T cells rendered dysfunctional by the tumor microenvironment (NCT01953900). Clin Cancer Res; 23(14); 3499-509. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures
References
-
- Gilham DE, et al. CAR-T cells and solid tumors: tuning T cells to challenge an inveterate foe. Trends Mol Med. 2012;18(7):377–84. - PubMed
-
- Rossig C, et al. Epstein-Barr virus-specific human T lymphocytes expressing antitumor chimeric T-cell receptors: potential for improved immunotherapy. Blood. 2002;99(6):2009–2016. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

