Ca2+-binding protein 2 inhibits Ca2+-channel inactivation in mouse inner hair cells
- PMID: 28183797
- PMCID: PMC5338518
- DOI: 10.1073/pnas.1617533114
Ca2+-binding protein 2 inhibits Ca2+-channel inactivation in mouse inner hair cells
Abstract
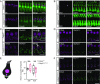
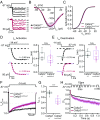
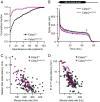
Ca2+-binding protein 2 (CaBP2) inhibits the inactivation of heterologously expressed voltage-gated Ca2+ channels of type 1.3 (CaV1.3) and is defective in human autosomal-recessive deafness 93 (DFNB93). Here, we report a newly identified mutation in CABP2 that causes a moderate hearing impairment likely via nonsense-mediated decay of CABP2-mRNA. To study the mechanism of hearing impairment resulting from CABP2 loss of function, we disrupted Cabp2 in mice (Cabp2LacZ/LacZ ). CaBP2 was expressed by cochlear hair cells, preferentially in inner hair cells (IHCs), and was lacking from the postsynaptic spiral ganglion neurons (SGNs). Cabp2LacZ/LacZ mice displayed intact cochlear amplification but impaired auditory brainstem responses. Patch-clamp recordings from Cabp2LacZ/LacZ IHCs revealed enhanced Ca2+-channel inactivation. The voltage dependence of activation and the number of Ca2+ channels appeared normal in Cabp2LacZ/LacZ mice, as were ribbon synapse counts. Recordings from single SGNs showed reduced spontaneous and sound-evoked firing rates. We propose that CaBP2 inhibits CaV1.3 Ca2+-channel inactivation, and thus sustains the availability of CaV1.3 Ca2+ channels for synaptic sound encoding. Therefore, we conclude that human deafness DFNB93 is an auditory synaptopathy.
Keywords: Ca2+ channel; hearing impairment; inner hair cell; ribbon synapse; synaptopathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

