Redox-based reagents for chemoselective methionine bioconjugation
- PMID: 28183972
- PMCID: PMC5827972
- DOI: 10.1126/science.aal3316
Redox-based reagents for chemoselective methionine bioconjugation
Abstract
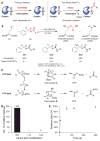
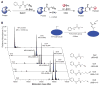
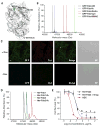
Cysteine can be specifically functionalized by a myriad of acid-base conjugation strategies for applications ranging from probing protein function to antibody-drug conjugates and proteomics. In contrast, selective ligation to the other sulfur-containing amino acid, methionine, has been precluded by its intrinsically weaker nucleophilicity. Here, we report a strategy for chemoselective methionine bioconjugation through redox reactivity, using oxaziridine-based reagents to achieve highly selective, rapid, and robust methionine labeling under a range of biocompatible reaction conditions. We highlight the broad utility of this conjugation method to enable precise addition of payloads to proteins, synthesis of antibody-drug conjugates, and identification of hyperreactive methionine residues in whole proteomes.
Copyright © 2017, American Association for the Advancement of Science.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

