Multiparametric Imaging of Tumor Hypoxia and Perfusion with 18F-Fluoromisonidazole Dynamic PET in Head and Neck Cancer
- PMID: 28183993
- PMCID: PMC5493008
- DOI: 10.2967/jnumed.116.188649
Multiparametric Imaging of Tumor Hypoxia and Perfusion with 18F-Fluoromisonidazole Dynamic PET in Head and Neck Cancer
Abstract
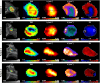
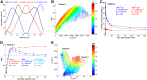
Tumor hypoxia and perfusion are independent prognostic indicators of patient outcome. We developed the methodology for and investigated the utility of multiparametric imaging of tumor hypoxia and perfusion with 18F-fluoromisonidazole (18F-FMISO) dynamic PET (dPET) in head and neck cancer. Methods: One hundred twenty head and neck cancer patients underwent 0- to 30-min 18F-FMISO dPET in a customized immobilization mask, followed by 10-min static acquisitions starting at 93 ± 6 and 160 ± 13 min after injection. A total of 248 lesions (≥2 cm3) were analyzed. Voxelwise pharmacokinetic modeling was conducted using an irreversible 1-plasma 2-tissue-compartment model to calculate surrogate biomarkers of tumor hypoxia (k3), perfusion (K1), and 18F-FMISO distribution volume. The analysis was repeated with truncated dPET datasets. Results: Substantial inter- and intratumor heterogeneity was observed for all investigated metrics. Equilibration between the blood and unbound 18F-FMISO was rapid in all tumors. 18F-FMISO distribution volume deviated from the expected value of unity, causing discrepancy between k3 maps and total 18F-FMISO uptake and reducing the dynamic range of total 18F-FMISO uptake for quantifying the degree of hypoxia. Both positive and negative trends between hypoxia and perfusion were observed in individual lesions. All investigated metrics were reproducible when calculated from a truncated 20-min dataset. Conclusion:18F-FMISO dPET provides the data necessary to generate parametric maps of tumor hypoxia, perfusion, and radiotracer distribution volume. These data clarify the ambiguity in interpreting 18F-FMISO uptake and improve the characterization of lesions. We show total acquisition times can be reduced to 20 min, facilitating the translation of 18F-FMISO dPET into the clinic.
Keywords: 18F-fluoromisonidazole; FMISO; dynamic PET; head and neck cancer; hypoxia; perfusion.
© 2017 by the Society of Nuclear Medicine and Molecular Imaging.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
