Phosphatidylserine Sensing by TAM Receptors Regulates AKT-Dependent Chemoresistance and PD-L1 Expression
- PMID: 28184013
- PMCID: PMC8363069
- DOI: 10.1158/1541-7786.MCR-16-0350
Phosphatidylserine Sensing by TAM Receptors Regulates AKT-Dependent Chemoresistance and PD-L1 Expression
Abstract
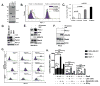
Tyro3, Axl, and Mertk (collectively TAM receptors) are three homologous receptor tyrosine kinases that bind vitamin K-dependent endogenous ligands, Protein S (ProS), and growth arrest-specific factor 6 (Gas6), and act as bridging molecules to promote phosphatidylserine (PS)-mediated clearance of apoptotic cells (efferocytosis). TAM receptors are overexpressed in a vast array of tumor types, whereby the level of expression correlates with the tumor grade and the emergence of chemo- and radioresistance to targeted therapeutics, but also have been implicated as inhibitory receptors on infiltrating myeloid-derived cells in the tumor microenvironment that can suppress host antitumor immunity. In the present study, we utilized TAM-IFNγR1 reporter lines and expressed TAM receptors in a variety of epithelial cell model systems to show that each TAM receptor has a unique pattern of activation by Gas6 or ProS, as well as unique dependency for PS on apoptotic cells and PS liposomes for activity. In addition, we leveraged this system to engineer epithelial cells that express wild-type TAM receptors and show that although each receptor can promote PS-mediated efferocytosis, AKT-mediated chemoresistance, as well as upregulate the immune checkpoint molecule PD-L1 on tumor cells, Mertk is most dominant in the aforementioned pathways. Functionally, TAM receptor-mediated efferocytosis could be partially blocked by PS-targeting antibody 11.31 and Annexin V, demonstrating the existence of a PS/PS receptor (i.e., TAM receptor)/PD-L1 axis that operates in epithelial cells to foster immune escape. These data provide a rationale that PS-targeting, anti-TAM receptor, and anti-PD-L1-based therapeutics will have merit as combinatorial checkpoint inhibitors.Implications: Many tumor cells are known to upregulate the immune checkpoint inhibitor PD-L1. This study demonstrates a role for PS and TAM receptors in the regulation of PD-L1 on cancer cells. Mol Cancer Res; 15(6); 753-64. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Cyril Empig, Bruce Freimark, Michael Gray and Jeff Hutchins are employees of Peregrine Pharmaceuticals, Inc. and have financial interest in the company. Allen Krantz and Andrzej Wilczynski are employees of Advanced Proteome Therapeutics Corporation. Allen Krantz has a financial interest in the company. Raymond B. Birge reports receiving a commercial research grant from Peregrine Pharmaceuticals, Inc. No potential conflicts of interest were disclosed by the other authors.
Figures





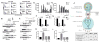
References
-
- Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–57. - PubMed
-
- Graham DK, Dawson TL, Mullaney DL, Snodgrass HR, Earp HS. Cloning and mRNA expression analysis of a novel human protooncogene, c-mer. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 1994;5:647–57. - PubMed
-
- Kumar S, Birge RB. Efferocytosis. Current biology : CB. 2016;26:R558–r9. - PubMed
-
- Prasad D, Rothlin CV, Burrola P, Burstyn-Cohen T, Lu Q, Garcia de Frutos P, et al. TAM receptor function in the retinal pigment epithelium. Mol Cell Neurosci. 2006;33:96–108. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

