Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: pharmacokinetics and biodistribution profile
- PMID: 28184163
- PMCID: PMC5291330
- DOI: 10.2147/IJN.S121881
Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: pharmacokinetics and biodistribution profile
Abstract
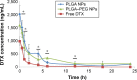
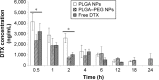
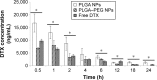
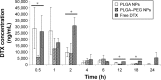
Docetaxel is a highly potent anticancer agent being used in a wide spectrum of cancer types. There are important matters of concern regarding the drug's pharmacokinetics related to the conventional formulation. Poly(lactide-co-glycolide) (PLGA) is a biocompatible/biodegradable polymer with variable physicochemical characteristics, and its application in human has been approved by the United States Food and Drug Administration. PLGA gives polymeric nanoparticles with unique drug delivery characteristics. The application of PLGA nanoparticles (NPs) as intravenous (IV) sustained-release delivery vehicles for docetaxel can favorably modify pharmacokinetics, biofate, and pharmacotherapy of the drug in cancer patients. Surface modification of PLGA NPs with poly(ethylene glycol) (PEG) can further enhance NPs' long-circulating properties. Herein, an optimized fabrication approach has been used for the preparation of PLGA and PLGA-PEG NPs loaded with docetaxel for IV application. Both types of NP formulations demonstrated in vitro characteristics that were considered suitable for IV administration (with long-circulating sustained-release purposes). NP formulations were IV administered to an animal model, and docetaxel's pharmacokinetic and biodistribution profiles were determined and compared between study groups. PLGA and PEGylated PLGA NPs were able to modify the pharmacokinetics and biodistribution of docetaxel. Accordingly, the mode of changes made to pharmacokinetics and biodistribution of docetaxel is attributed to the size and surface properties of NPs. NPs contributed to increased blood residence time of docetaxel fulfilling their role as long-circulating sustained-release drug delivery systems. Surface modification of NPs contributed to more pronounced docetaxel blood concentration, which confirms the role of PEG in conferring long-circulation properties to NPs.
Keywords: biodistribution; docetaxel; emulsification solvent evaporation; pharmacokinetics; poly(lactide-co-glycolide); poly(lactide-co-glycolide)-poly(ethylene glycol); polymeric nanoparticles; sustained release.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
-
- Cooper GM. Elements of Human Cancer. Burlington, MA: Jones and Bartlett Publishers Inc; 1992. Chapter 2: classification and development of neoplasms; pp. 15–30.
-
- Canadian Cancer Society . Canadian Cancer Statistics. Toronto, ON: Canadian Cancer Society; 2015.
-
- Chidambaram M, Manavalan R, Kathiresan K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J Pharm Pharm Sci. 2011;14(1):67–77. - PubMed
-
- Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumortargeted drug delivery based on EPR-effect. Eur J Pharm Biopharm. 2009;71(3):409–419. - PubMed
-
- Feng S-S, Chien S. Chemotherapeutic engineering: application and further development of chemical engineering principles for chemotherapy of cancer and other diseases. Chem Eng Sci. 2003;58(18):4087–4114.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

