A Systematic Relationship Between Functional Connectivity and Intracortical Myelin in the Human Cerebral Cortex
- PMID: 28184415
- PMCID: PMC5390400
- DOI: 10.1093/cercor/bhx030
A Systematic Relationship Between Functional Connectivity and Intracortical Myelin in the Human Cerebral Cortex
Abstract
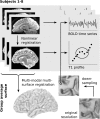
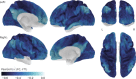
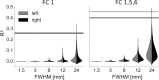
Research in the macaque monkey suggests that cortical areas with similar microstructure are more likely to be connected. Here, we examine this link in the human cerebral cortex using 2 magnetic resonance imaging (MRI) measures: quantitative T1 maps, which are sensitive to intracortical myelin content and provide an in vivo proxy for cortical microstructure, and resting-state functional connectivity. Using ultrahigh-resolution MRI at 7 T and dedicated image processing tools, we demonstrate a systematic relationship between T1-based intracortical myelin content and functional connectivity. This effect is independent of the proximity of areas. We employ nonlinear dimensionality reduction to characterize connectivity components and identify specific aspects of functional connectivity that are linked to myelin content. Our results reveal a consistent spatial pattern throughout different analytic approaches. While functional connectivity and myelin content are closely linked in unimodal areas, the correspondence is lower in transmodal areas, especially in posteromedial cortex and the angular gyrus. Our findings are in agreement with comprehensive reports linking histologically assessed microstructure and connectivity in different mammalian species and extend them to the human cerebral cortex in vivo.
Keywords: cortical microstructure; functional connectivity; high-resolution MRI; myelin imaging.
© The Author 2017. Published by Oxford University Press.
Figures




References
-
- Adachi Y, Osada T, Sporns O, Watanabe T, Matsui T, Miyamoto K, Miyashita Y. 2012. Functional connectivity between anatomically unconnected areas is shaped by collective network-level effects in the macaque cortex. Cereb Cortex. 22(7):1586–1592. - PubMed
-
- Annese J, Pitiot A, Dinov ID, Toga AW. 2004. A myelo-architectonic method for the structural classification of cortical areas. Neuroimage. 21(1):15–26. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

