Regulation of PD-L1 expression on murine tumor-associated monocytes and macrophages by locally produced TNF-α
- PMID: 28184968
- PMCID: PMC6469500
- DOI: 10.1007/s00262-017-1955-5
Regulation of PD-L1 expression on murine tumor-associated monocytes and macrophages by locally produced TNF-α
Abstract
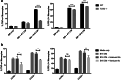
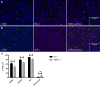
PD-L1 is an immune checkpoint protein that has emerged as a major signaling molecule involved with tumor escape from T cell immune responses. Studies have shown that intra-tumoral expression of PD-L1 can inhibit antitumor immune responses. However, it has recently been shown that expression of PD-L1 on myeloid cells from the tumor is a stronger indicator of prognosis than tumor cell PD-L1 expression. Therefore, it is important to understand the factors that govern the regulation of PD-L1 expression on tumor-infiltrating myeloid cells. We found that immature bone marrow monocytes in tumor-bearing mice had low levels of PD-L1 expression, while higher levels of expression were observed on monocytes in circulation. In contrast, macrophages found in tumor tissues expressed much higher levels of PD-L1 than circulating monocytes, implying upregulation by the tumor microenvironment. We demonstrated that tumor-conditioned media strongly induced increased PD-L1 expression by bone marrow-derived monocytes and TNF-α to be a cytokine that causes an upregulation of PD-L1 expression by the monocytes. Furthermore, we found production of TNF-α by the monocytes themselves to be a TLR2-dependent response to versican secreted by tumor cells. Thus, PD-L1 expression by tumor macrophages appears to be regulated in a different manner than by tumor cells themselves.
Keywords: Cytokine; Immune checkpoint; Myeloid; Tumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Tarin D, Price JE. Influence of microenvironment and vascular anatomy on “metastatic” colonization potential of mammary tumors. Cancer Res. 1981;41(9 Pt 1):3604–3609. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

