Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD)
- PMID: 28185242
- PMCID: PMC6464543
- DOI: 10.1002/14651858.CD012066.pub2
Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD)
Update in
-
Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2023 Jun 5;6(6):CD012066. doi: 10.1002/14651858.CD012066.pub3. Cochrane Database Syst Rev. 2023. PMID: 37276335 Free PMC article.
Abstract
Background: Three classes of inhaler medications are used to manage chronic obstructive pulmonary disease (COPD): long-acting beta-agonists (LABA), long-acting muscarinic antagonists (LAMA), and inhaled corticosteroids (ICS). When two classes of medications are required, LAMA plus LABA (LAMA+LABA) and LABA plus ICS (LABA+ICS) are often selected because these combinations can be administered via a single medication device. The previous Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidance recommended LABA+ICS as the first-line treatment for managing stable COPD in high-risk people of categories C and D. However, the updated GOLD 2017 guidance recommends LAMA+LABA over LABA+ICS.
Objectives: To compare the benefits and harms of LAMA+LABA versus LABA+ICS for treatment of people with stable COPD.
Search methods: We performed an electronic search of the Cochrane Airways Group Specialised Register (2 February 2016), ClinicalTrials.gov (4 June 2016), and the World Health Organization Clinical Trials Search Portal (4 June 2016), followed by a handsearch (5 June 2016). Two review authors screened and scrutinised the selected articles.
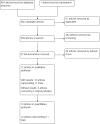
Selection criteria: We included individual randomised controlled trials, parallel-group trials, and cross-over trials comparing LAMA+LABA and LABA+ICS for stable COPD. The minimum accepted trial duration was one month and trials should have been conducted in an outpatient setting.
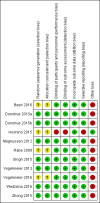
Data collection and analysis: Two review authors independently extracted data and evaluated risk of bias. We resolved any discrepancies through discussion. We analysed dichotomous data as odds ratios (OR), and continuous data as mean differences (MD), with 95% confidence interval (CI) using Review Manager 5. Exacerbations were measured by counting the number of people experiencing one or more exacerbation.
Main results: We included 11 studies comprising 9839 participants in our quantitative analysis. Most studies included people with moderate to severe COPD, without recent exacerbations. One pharmaceutical sponsored trial that included only people with recent exacerbations was the largest study and accounted for 37% of participants. All but one study were sponsored by pharmaceutical companies, thus we rated them as having a high risk of 'other bias'. The unsponsored study was at high risk of performance and detection bias, and possible selective reporting.Five studies recruited GOLD Category B participants, one study recruited Category D participants, two studies recruited Category A/B participants, and three studies recruited participants regardless of category. Follow-up ranged from 6 to 52 weeks.Compared to the LABA+ICS arm, the results for the pooled primary outcomes for the LAMA+LABA arm were as follows: exacerbations, OR 0.82 (95% CI 0.70 to 0.96, P = 0.01, I2 = 17%, low quality evidence); serious adverse events (SAE), OR 0.91 (95% CI 0.79 to 1.05, P = 0.18, I2 = 0, moderate quality evidence); St. George's Respiratory Questionnaire (SGRQ) total score change from the baseline, MD -1.22 (95% CI -2.52 to 0.07, P = 0.06, I2 = 71%, low quality evidence); and trough forced expiratory volume in one second (FEV1) change from the baseline, MD 0.08 L (95% CI 0.06 to 0.09, P < 0.0001, I2 = 50%, moderate quality evidence). Compared to the LABA+ICS arm, the results for the pooled secondary outcomes for the LAMA+LABA arm were as follows: pneumonia, OR 0.57 (95% CI 0.42 to 0.79, P = 0.0006, I2 = 0%, low quality evidence); all-cause death, OR 1.01 (95% CI 0.61 to 1.67, P = 0.88, I2 = 0%, low quality evidence); and SGRQ total score change from the baseline of 4 points or greater (the minimal clinically important difference for the SGRQ is 4 points), OR 1.25 (95% CI 1.09 to 1.44, P = 0.002, I2 = 0%, moderate quality evidence).
Authors' conclusions: For the treatment of COPD, LAMA+LABA has fewer exacerbations, a larger improvement of FEV1, a lower risk of pneumonia, and more frequent improvement in quality of life as measured by an increase over 4 units or more of the SGRQ. These data were supported by low or moderate quality evidence generated from mainly participants with moderate to severe COPD in heterogeneous trials with an observation period of less than one year. Our findings support the recently updated GOLD guidance.
Conflict of interest statement
TK: received grants or lecture fees (or both) from GlaxoSmithKline, Boehringer Ingelheim, Pfizer, Meiji, AstraZeneca, and Novartis from 2013 to 2016. This review was not funded by any pharmaceutical companies.
HN, GA, SY, OE, NK, and NK: none known.
Figures












Comment in
-
In stable COPD, long-acting muscarinic antagonist plus long-acting beta-agonists resulted in less exacerbations, pneumonia and larger improvement in FEV 1 than long-acting beta-agonists plus inhaled corticosteroids.Evid Based Med. 2017 Oct;22(5):183-184. doi: 10.1136/ebmed-2017-110726. Epub 2017 Aug 11. Evid Based Med. 2017. PMID: 28801320 No abstract available.
References
References to studies included in this review
Beeh 2016 {published data only}
-
- Beeh KM, Derom E, Echave‐Sustaeta J, Grönke L, Hamilton A, Zhai D, et al. The lung function profile of once‐daily tiotropium and olodaterol via Respimat is superior to that of twice‐daily salmeterol and fluticasone propionate via Accuhaler (ENERGITO study). International Journal of Chronic Obstructive Pulmonary Disease 2016;11:193‐205. [PUBMED: 26893551] - PMC - PubMed
Donohue 2015a {published data only (unpublished sought but not used)}
-
- Donohue J, Worsley S, Zhu CQ, Hardaker L, Church A. Efficacy and safety of umeclidinium/vilanterol (UMEC/VI) once daily (OD) vs fluticasone/salmeterol combination (FSC) twice daily (BD) in patients with moderate‐to‐severe COPD and infrequent COPD exacerbations. Chest 2014;146(4):73A.
-
- Donohue JF, Worsley S, Zhu CQ, Hardaker L, Church A. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate‐to‐severe COPD and infrequent exacerbations. Respiratory Medicine 2015;109(7):870‐81. [PUBMED: 26006754] - PubMed
Donohue 2015b {published data only (unpublished sought but not used)}
-
- Donohue J, Worsley S, Zhu CQ, Hardaker L, Church A. Efficacy and safety of umeclidinium/vilanterol (UMEC/VI) once daily (OD) vs fluticasone/salmeterol combination (FSC) twice daily (BD) in patients with moderate‐to‐severe COPD and infrequent COPD exacerbations. Chest 2014;146(4):73A.
-
- Donohue JF, Worsley S, Zhu CQ, Hardaker L, Church A. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate‐to‐severe COPD and infrequent exacerbations. Respiratory Medicine 2015;109(7):870‐81. [PUBMED: 26006754] - PubMed
Hoshino 2015 {published data only}
-
- Hoshino M, Ohtawa J, Akitsu K. Comparison of airway dimensions with once daily tiotropium plus indacaterol versus twice daily Advair((R)) in chronic obstructive pulmonary disease. Pulmonary Pharmacology & Therapeutics 2015;30:128‐33. [PUBMED: 25183687] - PubMed
Magnussen 2012 {published data only}
-
- Magnussen H, Maltais F, Schmidt H, Kesten S, Metzdorf N. Comparison of tiotropium+salmeterol vs. fluticasone+salmeterol on lung volumes, exercise tolerance and locus of symptom limitation. American Journal of Respiratory and Critical Care Medicine 2010;181:A4472.
-
- Magnussen H, Paggiaro P, Schmidt H, Kesten S, Metzdorf N, Maltais F. Effect of combination treatment on lung volumes and exercise endurance time in COPD. Respiratory Medicine 2012;106(10):1413‐20. [PUBMED: 22749044] - PubMed
Rabe 2008 {published data only (unpublished sought but not used)}
-
- Rabe KF, Timmer W, Sagkriotis A, Viel K. Comparison of a combination of tiotropium plus formoterol to salmeterol plus fluticasone in moderate COPD. Chest 2008;134(2):255‐62. [PUBMED: 18403672] - PubMed
-
- Rabe KF, Timmer W, Sagriotis A, Viel K. Comparison of a combination of tiotropium and formoterol to salmeterol and fluticasone in moderate COPD. 15th European Respiratory Society Annual Congress; 2005 Sept 30‐31; Copenhagen, Denmark. 2005.
Singh 2015 {published data only (unpublished sought but not used)}
-
- Singh D, Worsley S, Zhu CQ, Hardaker L, Church A. Umeclidinium/vilanterol (UMEC/VI) once daily (OD) vs fluticasone/salmeterol combination (FSC) twice daily (BD) in patients with moderate‐to‐severe COPD and infrequent COPD exacerbations. European Respiratory Journal 2014;44(Suppl 58):P290.
Vogelmeier 2013 {published data only (unpublished sought but not used)}
-
- Bateman E, Vogelmeier C, Chen H, Banerji D. Comparison of COPD exacerbations with once‐daily QVA149 versus twice‐daily salmeterol/fluticasone combination: the ILLUMINATE study. American College of Chest Physicians 2014;145(3):409A.
-
- Bateman ED, Vogelmeier C, Pallante J, Bryant H, Alagappan V, D'Andrea P, et al. Once‐daily QVA149 demonstrates superior lung function compared to twice‐daily salmeterol/fluticasone in all subgroups of COPD patients: the ILLUMINATE study. American Journal of Respiratory and Critical Care Medicine 2013;187:A4273.
-
- Bateman ED, Vogelmeier C, Pallante J, Bryant H, Alagappan V, D'Andrea P, et al. Once‐daily QVA149 improves breathlessness and reduces rescue medication use compared to twice‐daily salmeterol/fluticasone in patients with COPD: the ILLUMINATE study. American Journal of Respiratory and Critical Care Medicine 2013;187:A2433.
-
- Mezzi K, Pallante J, Alagappan V, Chen H, Banerji D. Once‐daily QVA149 demonstrates superior outcomes in COPD patients previously treated with fixed‐dose long‐acting β2‐agonist/inhaled corticosteroid (LABA/ICS): the ILLUMINATE study. Chest 2014;145(3):424A.
-
- Vogelmeier C, Bateman E, Pallante J, Bryant H, Alagappan VD, Andrea P, et al. Once‐daily QVA149 provides superior bronchodilation and improves lung function versus twice‐daily fluticasone/salmeterol in COPD patients: the ILLUMINATE study. British Thoracic Society Winter Meeting 2012;67:A149, P194.
Vogelmeier 2016 {published data only (unpublished sought but not used)}
-
- Vogelmeier C, Paggiaro PL, Dorca J, Sliwinski P, Mallet M, Kirsten A, et al. The efficacy and safety of aclidinium/formoterol fixed‐dose combination compared with salmeterol/fluticasone in patients with COPD: results from a phase III study. American Journal of Respiratory and Critical Care Medicine 2015;191:A3974.
-
- Vogelmeier C, Paggiaro PL, Dorca J, Sliwinski P, Mallet M, Kirsten AM, et al. Efficacy and safety of aclidinium/formoterol versus salmeterol/fluticasone: a phase 3 COPD study. European Respiratory Journal 2016; Vol. 48, issue 4:1030‐9. - PubMed
Wedzicha 2016 {published data only (unpublished sought but not used)}
-
- Kwaijtaal M, Wedzicha J, Decramer M, Vestbo J, Banerji D. A novel study design for the comparison between once‐daily QVA149 and twice‐daily salmeterol/fluticasone on the reduction of COPD exacerbations: the FLAME study. Respirology 2014:TP177.
-
- Wedzicha J, Vestbo J, Gallagher N, Banerji D. A novel study design for the comparison between once‐daily QVA149 and twice‐daily salmeterol/fluticasone on the reduction of COPD exacerbations: the FLAME study. Chest 2014;145(3):408A.
-
- Wedzicha JA, Banerji D, Chapman KR, Vestbo J, Roche N, Ayers RT, et al. Indacaterol‐glycopyrronium versus salmeterol‐fluticasone for COPD. New England Journal of Medicine 2016;374(23):2222‐34. [PUBMED: 27181606] - PubMed
Zhong 2015 {published data only (unpublished sought but not used)}
References to studies excluded from this review
Bruhn 2003 {published data only}
-
- Bruhn C. Chronic obstructive pulmonary disease: recommendation of salmeterol in fixed combination. Deutsche Apotheker‐Zeitung 2003;143(11):48‐51.
Calverley 2007 {published data only}
-
- Calverley P, Stockley R, Seemungal T, Hagan G, Wedzicha J. Adverse events and mortality in the INSPIRE study (Investigating New Standards for Prophylaxis In Reduction of Exacerbations). 17th European Respiratory Society Annual Congress 2007;30:125s [P847]. - PubMed
Knobil 2004a {published data only}
-
- Knobil K, Kalberg C, Merchant K, Emmett A, Cicale M. Maintenance of bronchodilator response for Advair Diskus 250/50 (fluticasone propionate/salmeterol) but not ipratropium/albuterol in patients with COPD. Chest 2004;126(Suppl 4):807S.
Knobil 2004b {published data only}
-
- Knobil K, Merchant K, Kalberg C, Emmett A, Cicale M. A comparison of patient perceived improvement in symptoms after initiating therapy with either Advair Diskus (fluticasone propionate/salmeterol) 250/50 or ipratropium/albuterol. Chest 2004;126(Suppl 4):806S‐b‐807S‐b.
NCT00120978 {published data only}
-
- NCT00120978. Can advair and flovent reduce systemic inflammation related to chronic obstructive pulmonary disease (COPD)? A multi‐center randomized controlled trial. clinicaltrials.gov/ct2/show/NCT00120978 Date first received: 11 July 2005.
Price 2014 {published data only}
-
- Price D, Keininger D, Costa‐Scharplatz M, Mezzi K, Dimova M, Asukai Y, et al. Cost‐effectiveness of the LABA/LAMA dual bronchodilator indacaterol/glycopyrronium in a Swedish healthcare setting. Respiratory Medicine 2014;108(12):1786‐93. - PubMed
Sciurba 2004 {published data only}
-
- Sciurba FC, Kalberg C, Emmett A, Merchant K, Brown C, Knobil K. Efficacy of Advair Diskus 250/50 (fluticasone propionate/salmeterol) or ipratropium/albuterol in patients with COPD associated with chronic bronchitis and/or emphysema. Chest 2004;126(Suppl 4):807S‐a‐808S‐a.
References to ongoing studies
NCT02497001 {published data only}
-
- NCT02497001. A randomized, double‐blind, parallel‐group, 24‐week, chronic‐dosing, multi‐center study to assess the efficacy and safety of PT010, PT003, and PT009 compared with Symbicort® Turbuhaler® (Kronos). clinicaltrials.gov/show/NCT02497001 Date first received: 8 July 2015.
NCT02516592 {published data only}
-
- NCT02516592. Assessment of switching from salmeterol/fluticasone to indacaterol/glycopyrronium in a symptomatic COPD patient cohort (FLASH). Date first received: 4 August 2015.
Additional references
Alagha 2014
Anderson 2014
Barnes 2010
-
- Barnes PJ. Inhaled corticosteroids in COPD: a controversy. Respiration 2010;80(2):89‐95. - PubMed
Burrows 1966
-
- Burrows B, Fletcher CM, Heard BE, Jones NL, Wootliff JS. The emphysematous and bronchial types of chronic airways obstruction. A clinicopathological study of patients in London and Chicago. Lancet 1966;1(7442):830‐5. - PubMed
Celli 2014
-
- Celli B, Crater G, Kilbride S, Mehta R, Tabberer M, Kalberg C, et al. Once‐daily umeclidinium/vilanterol 125/25 mcg in COPD: a randomized, controlled study. Chest 2014;145(5):981‐91. - PubMed
Cluzeau 2012
-
- Cluzeau F, Wedzicha JA, Kelson M, Corn J, Kunz R, Walsh J, et al. Stakeholder involvement: how to do it right: article 9 in Integrating and coordinating efforts in COPD guideline development. An official ATS/ERS workshop report. Proceedings of the American Thoracic Society Journal 2012;9(5):269‐73. - PubMed
Drivenes 2014
Frampton 2014
-
- Frampton JE. QVA149 (indacaterol/glycopyrronium fixed‐dose combination): a review of its use in patients with chronic obstructive pulmonary disease. Drugs 2014;74(4):465‐88. - PubMed
GOLD 2016
-
- The Global Initiative for Chronic Obstructive Lung Disease. From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016. www.goldcopd.org/Guidelines/guidelines‐resources.html (accessed 16 August 2016).
GOLD 2017
-
- The Global Initiative for Chronic Obstructive Lung Disease. From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. www.goldcopd.org/Guidelines/guidelines‐resources.html (accessed 7 December 2016).
GRADEpro [Computer program]
-
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. Hamilton (ON): McMaster University, 2008.
Guyatt 2008
Hanania 2008
-
- Hanania NA. The impact of inhaled corticosteroid and long‐acting beta‐agonist combination therapy on outcomes in COPD. Pulmonary Pharmacology & Therapeutics 2008;21(3):540‐50. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Horita 2015a
Horita 2015b
-
- Horita N, Miyazawa N, Tomaru K, Inoue M, Kaneko T. Long‐acting muscarinic antagonist + long‐acting beta agonist versus long‐acting beta agonist + inhaled corticosteroid for COPD: a systematic review and meta‐analysis. Respirology 2015;20(8):1153‐9. - PubMed
Malerba 2014
Moher 2009
Nannini 2012
-
- Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long‐acting beta2‐agonist in one inhaler versus long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD006829.pub2] - DOI
Nannini 2013
Oba 2016
-
- Oba Y, Chandran AV, Devasahayam JV. Long‐acting muscarinic antagonist versus inhaled corticosteroid when added to long‐acting β‐agonist for COPD: a meta‐analysis. COPD 2016;13(6):677‐85. - PubMed
Pascoe 2015
-
- Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respiratory Medicine 2015;3(6):435‐42. - PubMed
Pauwels 2001
-
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS, GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. American Journal of Respiratory and Critical Care Medicine 2001;163(5):1256‐76. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schachter 2013
-
- Schachter EN. Indacaterol/glycopyrronium bromide fixed‐dose combination for the treatment of COPD. Drugs Today (Barcelona, Spain : 1998) 2013;49(7):437‐46. - PubMed
Suissa 2009
-
- Suissa S, Barnes PJ. Inhaled corticosteroids in COPD: the case against. European Respiratory Journal 2009;34(1):13‐6. - PubMed
Tashkin 2004
-
- Tashkin DP, Cooper CB. The role of long‐acting bronchodilators in the management of stable COPD. Chest 2004;125(1):249‐59. - PubMed
White 2013
WHO 2015
-
- World Health Organization. Chronic respiratory disease: chronic obstructive pulmonary disease (COPD): burden of COPD. www.who.int/respiratory/copd/burden/en/ (accessed 31 October 2015).
References to other published versions of this review
Horita 2016
-
- Horita N, Goto A, Ota E, Nakashima K, Nagai K, Kaneko T. Long‐acting muscarinic antagonist plus long‐acting beta agonist versus long‐acting beta agonist plus inhaled corticosteroid for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD012066] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

