Customized biomaterials to augment chondrocyte gene therapy
- PMID: 28185909
- PMCID: PMC7707423
- DOI: 10.1016/j.actbio.2017.02.008
Customized biomaterials to augment chondrocyte gene therapy
Abstract
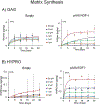
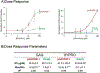
A persistent challenge in enhancing gene therapy is the transient availability of the target gene product. This is particularly true in tissue engineering applications. The transient exposure of cells to the product could be insufficient to promote tissue regeneration. Here we report the development of a new material engineered to have a high affinity for a therapeutic gene product. We focus on insulin-like growth factor-I (IGF-I) for its highly anabolic effects on many tissues such as spinal cord, heart, brain and cartilage. One of the ways that tissues store IGF-I is through a group of insulin like growth factor binding proteins (IGFBPs), such as IGFBP-5. We grafted the IGF-I binding peptide sequence from IGFBP-5 onto alginate in order to retain the endogenous IGF-I produced by transfected chondrocytes. This novel material bound IGF-I and released the growth factor for at least 30days in culture. We found that this binding enhanced the biosynthesis of transfected cells up to 19-fold. These data demonstrate the coordinated engineering of cell behavior and material chemistry to greatly enhance extracellular matrix synthesis and tissue assembly, and can serve as a template for the enhanced performance of other therapeutic proteins.
Statement of significance: The present manuscript focuses on the enhancement of chondrocyte gene therapy through the modification of scaffold materials to enhance the retention of targeted gene products. This study combined tissue engineering and gene therapy, where customized biomaterials augmented the action of IGF-I by enhancing the retention of protein produced by transfection of the IGF-I gene. This approach enabled tuning of binding of IGF-I to alginate, which increased GAG and HYPRO production by transfected chondrocytes. To our knowledge, peptide-based modification of materials to augment growth factor-targeted gene therapy has not been reported previously.
Keywords: Binding peptide; Growth factor; IGF-I; Osteoarthritis.
Copyright © 2017 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures


References
-
- Weisleder N, Takizawa N, Lin P, Wang X, Cao C, Zhang Y, Tan T, Ferrante C, Zhu H, Chen P-J, Yan R, Sterling M, Zhao X, Hwang M, Takeshima M, Cai C, Cheng H, Takeshima H, Xiao R-P, Ma J, Recombinant MG53 Protein Modulates Therapeutic Cell Membrane Repair in Treatment of Muscular Dystrophy, Sci. Transl. Med 4 (2012) 139ra85–139. doi: 10.1126/scitranslmed.3003921. - DOI - PMC - PubMed
-
- Nagahara AH, a Merrill D, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, V Chao M, Koo EH, Geschwind D, Masliah E, a Chiba A, Tuszynski MH, Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease., Nat. Med 15 (2009) 331–337. doi: 10.1038/nm.1912. - DOI - PMC - PubMed
-
- Bartus K, James ND, Didangelos A, Bosch KD, Verhaagen J, Yáñez-Muñoz RJ, Rogers JH, Schneider BL, Muir EM, Bradbury EJ, Large-Scale Chondroitin Sulfate Proteoglycan Digestion with Chondroitinase Gene Therapy Leads to Reduced Pathology and Modulates Macrophage Phenotype following Spinal Cord Contusion Injury., J. Neurosci 34 (2014) 4822–36. doi: 10.1523/JNEUROSCI.4369-13.2014. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

