Topical tacrolimus for the treatment of secondary lymphedema
- PMID: 28186091
- PMCID: PMC5309859
- DOI: 10.1038/ncomms14345
Topical tacrolimus for the treatment of secondary lymphedema
Abstract
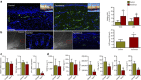
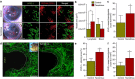
Secondary lymphedema, a life-long complication of cancer treatment, currently has no cure. Lymphedema patients have decreased quality of life and recurrent infections with treatments limited to palliative measures. Accumulating evidence indicates that T cells play a key role in the pathology of lymphedema by promoting tissue fibrosis and inhibiting lymphangiogenesis. Here using mouse models, we show that topical therapy with tacrolimus, an anti-T-cell immunosuppressive drug, is highly effective in preventing lymphedema development and treating established lymphedema. This intervention markedly decreases swelling, T-cell infiltration and tissue fibrosis while significantly increasing formation of lymphatic collaterals with minimal systemic absorption. Animals treated with tacrolimus have markedly improved lymphatic function with increased collecting vessel contraction frequency and decreased dermal backflow. These results have profound implications for lymphedema treatment as topical tacrolimus is FDA-approved for other chronic skin conditions and has an established record of safety and tolerability.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- DiSipio T., Rye S., Newman B. & Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 14, 500–515 (2013). - PubMed
-
- Petrek J. A. & Heelan M. C. Incidence of breast carcinoma-related lymphedema. Cancer 83, 2776–2781 (1998). - PubMed
-
- Rockson S. G. & Rivera K. K. Estimating the population burden of lymphedema. Ann. N. Y. Acad. Sci. 1131, 147–154 (2008). - PubMed
-
- Erickson V. S., Pearson M. L., Ganz P. A., Adams J. & Kahn K. L. Arm edema in breast cancer patients. J. Natl Cancer Inst. 93, 96–111 (2001). - PubMed
-
- Hayes S. C. et al.. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer 118, 2237–2249 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

