Dysregulated autophagy increased melanocyte sensitivity to H2O2-induced oxidative stress in vitiligo
- PMID: 28186139
- PMCID: PMC5301258
- DOI: 10.1038/srep42394
Dysregulated autophagy increased melanocyte sensitivity to H2O2-induced oxidative stress in vitiligo
Abstract
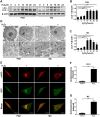
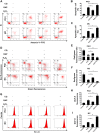
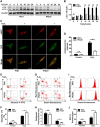
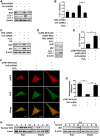
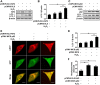
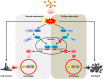
In vitiligo, melanocytes are particularly vulnerable to oxidative stress owing to the pro-oxidant state generated during melanin synthesis and to the genetic antioxidant defects. Autophagy is a controlled self-digestion process which can protect cells against oxidative damage. However, the exact role of autophagy in vitiligo melanocytes in response to oxidative stress and the mechanism involved are still not clear. To determine the implications of autophagy for melanocyte survival in response to oxidative stress, we first detected the autophagic flux in normal melanocytes exposure to H2O2, and found that autophagy was significantly enhanced in normal melanocytes, for protecting cells against H2O2-induced oxidative damage. Nevertheless, vitiligo melanocytes exhibited dysregulated autophagy and hypersensitivity to H2O2-induced oxidative injury. In addition, we confirmed that the impairment of Nrf2-p62 pathway is responsible for the defects of autophagy in vitiligo melanocytes. Noteworthily, upregulation of the Nrf2-p62 pathway or p62 reduced H2O2-induced oxidative damage of vitiligo melanocytes. Therefore, our data demonstrated that dysregulated autophagy owing to the impairment of Nrf2-p62 pathway increase the sensitivity of vitiligo melanocytes to oxidative stress, thus promote the development of vitiligo. Upregulation of p62-dependent autophagy may be applied to vitiligo treatment in the future.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Ongenae K. et al.. Psychosocial effects of vitiligo. J Eur Acad Dermatol Venereol 20, 1–8 (2006). - PubMed
-
- Boissy R. E. & Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res 17, 208–214 (2004). - PubMed
-
- Schallreuter K. U. et al.. Low catalase levels in the epidermis of patients with vitiligo. J Invest Dermatol 97, 1081–1085 (1991). - PubMed
-
- Schallreuter K. U. et al.. Blunted epidermal L-tryptophan metabolism in vitiligo affects immune response and ROS scavenging by Fenton chemistry, part 1: Epidermal H2O2/ONOO(−)-mediated stress abrogates tryptophan hydroxylase and dopa decarboxylase activities, leading to low serotonin and melatonin levels. FASEB J 26, 2457–2470 (2012). - PubMed
-
- Sravani P. V. et al.. Determination of oxidative stress in vitiligo by measuring superoxide dismutase and catalase levels in vitiliginous and non-vitiliginous skin. Indian J Dermatol Venereol Leprol 75, 268–271 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

