Selective molecular impairment of spontaneous neurotransmission modulates synaptic efficacy
- PMID: 28186166
- PMCID: PMC5311059
- DOI: 10.1038/ncomms14436
Selective molecular impairment of spontaneous neurotransmission modulates synaptic efficacy
Abstract
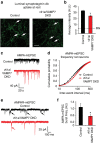
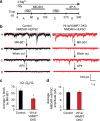
Recent studies suggest that stimulus-evoked and spontaneous neurotransmitter release processes are mechanistically distinct. Here we targeted the non-canonical synaptic vesicle SNAREs Vps10p-tail-interactor-1a (vti1a) and vesicle-associated membrane protein 7 (VAMP7) to specifically inhibit spontaneous release events and probe whether these events signal independently of evoked release to the postsynaptic neuron. We found that loss of vti1a and VAMP7 impairs spontaneous high-frequency glutamate release and augments unitary event amplitudes by reducing postsynaptic eukaryotic elongation factor 2 kinase (eEF2K) activity subsequent to the reduction in N-methyl-D-aspartate receptor (NMDAR) activity. Presynaptic, but not postsynaptic, loss of vti1a and VAMP7 occludes NMDAR antagonist-induced synaptic potentiation in an intact circuit, confirming the role of these vesicular SNAREs in setting synaptic strength. Collectively, these results demonstrate that spontaneous neurotransmission signals independently of stimulus-evoked release and highlight its role as a key regulator of postsynaptic efficacy.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Cannon W. B. & Rosenblueth A. The Supersensitivity of Denervated Structures: A Law of Denervation The Macmillan Company (1949).
-
- Turrigiano G. G., Leslie K. R., Desai N. S., Rutherford L. C. & Nelson S. B. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391, 892–896 (1998). - PubMed
-
- Sutton M. A. et al.. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell 125, 785–799 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

