Gene Transfer of Prolyl Hydroxylase Domain 2 Inhibits Hypoxia-inducible Angiogenesis in a Model of Choroidal Neovascularization
- PMID: 28186209
- PMCID: PMC5301234
- DOI: 10.1038/srep42546
Gene Transfer of Prolyl Hydroxylase Domain 2 Inhibits Hypoxia-inducible Angiogenesis in a Model of Choroidal Neovascularization
Abstract
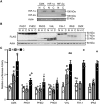
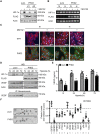
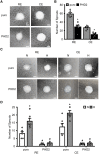
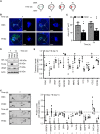
Cellular responses to hypoxia are mediated by the hypoxia-inducible factors (HIF). In normoxia, HIF-α proteins are regulated by a family of dioxygenases, through prolyl and asparagyl hydroxylation, culminating in proteasomal degradation and transcriptional inactivation. In hypoxia, the dioxygenases become inactive and allow formation of HIF transcription factor, responsible for upregulation of hypoxia genes. In ocular neoangiogenic diseases, such as neovascular age-related macular degeneration (nAMD), hypoxia seems pivotal. Here, we investigate the effects of HIF regulatory proteins on the hypoxia pathway in retinal pigment epithelium (RPE) cells, critically involved in nAMD pathogenesis. Our data indicates that, in ARPE-19 cells, prolyl hydroxylase domain (PHD)2 is the most potent negative-regulator of the HIF pathway. The negative effects of PHD2 on the hypoxia pathway were associated with decreased HIF-1α protein levels, and concomitant decrease in angiogenic factors. ARPE-19 cells stably expressing PHD2 impaired angiogenesis in vitro by wound healing, tubulogenesis, and sprouting assays, as well as in vivo by iris-induced angiogenesis. Gene transfer of PHD2 in vivo resulted in mitigation of HIF-mediated angiogenesis in a mouse model of nAMD. These results may have implications for the clinical treatment of nAMD patients, particularly regarding the use of gene therapy to negatively regulate neoangiogenesis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Ruas J. L. & Poellinger L. Hypoxia-dependent activation of HIF into a transcriptional regulator. Semin. Cell Dev. Biol. 16, 514–522 (2005). - PubMed
-
- Weidemann A. & Johnson R. S. Biology of HIF-1α. Cell Death Differ 15, 621–627 (2008). - PubMed
-
- Wang G. L. & Semenza G. L. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 270, 1230–1237 (1995). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

