Skin-Derived Precursors as a Source of Progenitors for Corneal Endothelial Regeneration
- PMID: 28186681
- PMCID: PMC5442762
- DOI: 10.1002/sctm.16-0162
Skin-Derived Precursors as a Source of Progenitors for Corneal Endothelial Regeneration
Abstract
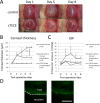
Corneal blindness is the fourth leading cause of blindness in the world. Current treatment is allogenic corneal transplantation, which is limited by shortage of donors and immunological rejection. Skin-derived precursors (SKPs) are postnatal stem cells, which are self-renewing, multipotent precursors that can be isolated and expanded from the dermis. Facial skin may therefore be an accessible autologous source of neural crest derived cells. SKPs were isolated from facial skin of Wnt1-Cre/Floxed EGFP mouse. After inducing differentiation with medium containing retinoic acid and GSK 3-β inhibitor, SKPs formed polygonal corneal endothelial-like cells (sTECE). Expression of major corneal endothelial markers were confirmed by Reverse transcription polymerase chain reaction (RT-PCR) and quantitative Real time polymerase chain reaction (qRT-PCR). Western blots confirmed the expression of Na, K-ATPase protein, the major functional marker of corneal endothelial cells. Immunohistochemistry revealed the expression of zonular occludens-1 and Na, K-ATPase in cell-cell junctions. In vitro functional analysis of Na, K-ATPase pump activity revealed that sTECE had significantly high pump function compared to SKPs or control 3T3 cells. Moreover, sTECE transplanted into a rabbit model of bullous keratopathy successfully maintained corneal thickness and transparency. Furthermore, we successfully induced corneal endothelial-like cells from human SKPs, and showed that transplanted corneas also maintained corneal transparency and thickness. Our findings suggest that SKPs may be used as a source of autologous cells for the treatment of corneal endothelial disease. Stem Cells Translational Medicine 2017;6:788-798.
Keywords: Cell culture; Cornea; Corneal endothelium; Neural crest; Skin progenitors.
© 2017 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures




References
-
- Joyce NC. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res 2003;22:359–389. - PubMed
-
- Hollingsworth J, Perez‐Gomez I, Mutalib HA et al. A population study of the normal cornea using an in vivo, slit‐scanning confocal microscope. Optom Vis Sci 2001;78:706–711. - PubMed
-
- Hatou S, Shimmura S, Shimazaki J et al. Mathematical projection model of visual loss due to fuchs corneal dystrophy. Invest Ophthalmol Vis Sci 2011;52:7888–7893. - PubMed
-
- Peh GS, Beuerman RW, Colman A et al. Human corneal endothelial cell expansion for corneal endothelium transplantation: An overview. Transplantation 2011;91:811–819. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

