Mechanisms of Tolerance Induction by Hematopoietic Chimerism: The Immune Perspective
- PMID: 28186688
- PMCID: PMC5442770
- DOI: 10.1002/sctm.16-0358
Mechanisms of Tolerance Induction by Hematopoietic Chimerism: The Immune Perspective
Abstract
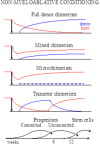
Hematopoietic chimerism is one of the effective approaches to induce tolerance to donor-derived tissue and organ grafts without administration of life-long immunosuppressive therapy. Although experimental efforts to develop such regimens have been ongoing for decades, substantial cumulative toxicity of combined hematopoietic and tissue transplants precludes wide clinical implementation. Tolerance is an active immunological process that includes both peripheral and central mechanisms of mutual education of coresident donor and host immune systems. The major stages include sequential suppression of early alloreactivity, establishment of hematopoietic chimerism and suppressor cells that sustain the state of tolerance, with significant mechanistic and temporal overlap along the tolerization process. Efforts to devise less toxic transplant strategies by reduction of preparatory conditioning focus on modulation rather than deletion of residual host immunity and early reinstitution of regulatory subsets at the central and peripheral levels. Stem Cells Translational Medicine 2017;6:700-712.
Keywords: Central tolerance; Hematopoietic cell transplants; Hematopoietic chimerism; Peripheral tolerance; Regulatory T cells; Transplant tolerance.
© 2017 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures



Similar articles
-
Mixed chimerism achieved by a nonlethal conditioning regimen induces donor-specific tolerance to lung allografts.J Surg Res. 2008 May 15;146(2):289-97. doi: 10.1016/j.jss.2007.07.017. Epub 2007 Aug 23. J Surg Res. 2008. PMID: 18314139
-
Application of xenogeneic stem cells for induction of transplantation tolerance: present state and future directions.Springer Semin Immunopathol. 2004 Nov;26(1-2):187-200. doi: 10.1007/s00281-004-0159-1. Epub 2004 Sep 11. Springer Semin Immunopathol. 2004. PMID: 15378269 Review.
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
-
Mixed hematopoietic chimerism and transplantation tolerance.Immunol Res. 1997;16(3):217-28. doi: 10.1007/BF02786391. Immunol Res. 1997. PMID: 9379073 Review.
-
Prevention of chronic rejection in murine cardiac allografts: a comparison of chimerism- and nonchimerism-inducing costimulation blockade-based tolerance induction regimens.J Immunol. 2002 Sep 1;169(5):2677-84. doi: 10.4049/jimmunol.169.5.2677. J Immunol. 2002. PMID: 12193741
Cited by
-
Cell therapy in vascularized composite allotransplantation.Biomed J. 2022 Jun;45(3):454-464. doi: 10.1016/j.bj.2022.01.005. Epub 2022 Jan 15. Biomed J. 2022. PMID: 35042019 Free PMC article. Review.
-
Lymphohematopoietic graft-versus-host responses promote mixed chimerism in patients receiving intestinal transplantation.J Clin Invest. 2021 Apr 15;131(8):e141698. doi: 10.1172/JCI141698. J Clin Invest. 2021. PMID: 33630757 Free PMC article.
-
Tolerance induction with donor hematopoietic stem cell infusion in kidney transplantation: a single-center experience in China with a 10-year follow-up.Ann Transl Med. 2020 Nov;8(21):1378. doi: 10.21037/atm-20-2502a. Ann Transl Med. 2020. PMID: 33313123 Free PMC article.
-
Accelerating cryoprotectant delivery using vacuum infiltration.Cryobiology. 2023 Sep;112:104558. doi: 10.1016/j.cryobiol.2023.104558. Epub 2023 Jul 13. Cryobiology. 2023. PMID: 37451668 Free PMC article.
-
Expansion of CD45RA-FOXP3++ regulatory T cells is associated with immune tolerance in patients with combined kidney and bone marrow transplantation.Clin Transl Immunology. 2021 Aug 9;10(8):e1325. doi: 10.1002/cti2.1325. eCollection 2021. Clin Transl Immunology. 2021. PMID: 34401148 Free PMC article.
References
-
- Wekerle T, Sykes M. Induction of tolerance. Surgery 2004;135:359–364. - PubMed
-
- Pearl‐Yafe M, Yolcu ES, Yaniv I et al. The dual role of Fas‐ligand as an injury effector and defense strategy in diabetes and islet transplantation. Bioessays 2006;28:211–222. - PubMed
-
- Metchkoff E, Roux E. Sur la propriété bactéricide du sang de rat. Ann Inst Pasteur 1891;5:479–486.
-
- Ehrlich P. Croonian lecture: On immunity with special reference to cell life. Proc R Soc London 1900;66:424–448.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

