Cumulative effects of neonatal hyperoxia on murine alveolar structure and function
- PMID: 28186703
- PMCID: PMC5621136
- DOI: 10.1002/ppul.23654
Cumulative effects of neonatal hyperoxia on murine alveolar structure and function
Abstract
Background: Bronchopulmonary dysplasia (BPD) results from alveolar simplification and abnormal development of alveolar and capillary structure. Survivors of BPD display persistent deficits in airflow and membrane and vascular components of alveolar gas diffusion. Despite being the defining feature of BPD, various neonatal hyperoxia models of BPD have not routinely assessed pulmonary gas diffusion.
Methods: To simulate the most commonly-utilized neonatal hyperoxia models, we exposed neonatal mice to room air or ≥90% hyperoxia during key stages of distal lung development: through the first 4 (saccular), 7 (early alveolar), or 14 (bulk alveolar) postnatal days, followed by a period of recovery in room air until 8 weeks of age when alveolar septation is essentially complete. We systematically assessed and correlated the effects of neonatal hyperoxia on the degree of alveolar-capillary structural and functional impairment. We hypothesized that the degree of alveolar-capillary simplification would correlate strongly with worsening diffusion impairment.
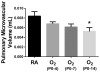
Results: Neonatal hyperoxia exposure, of any duration, resulted in alveolar simplification and impaired pulmonary gas diffusion. Mean Linear Intercept increased in proportion to the length of hyperoxia exposure while alveolar and total lung volume increased markedly only with prolonged exposure. Surprisingly, despite having a similar effect on alveolar surface area, only prolonged hyperoxia for 14 days resulted in reduced pulmonary microvascular volume. Estimates of alveolar and capillary structure, in general, correlated poorly with assessment of gas diffusion.
Conclusion: Our results help define the physiological and structural consequences of commonly-employed neonatal hyperoxia models of BPD and inform their clinical utility. Pediatr Pulmonol. 2017;52:616-624. © 2016 Wiley Periodicals, Inc.
Keywords: bronchopulmonary dysplasia; hyperoxia; lung function; neonatal; pulmonary diffusion capacity.
© 2016 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of interest: None.
Figures
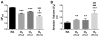
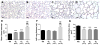
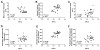
References
-
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sanchez PJ, O’Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID, III, Watterberg KL, Saha S, Das A, Higgins RD. For the eunice kennedy shriver national institute of child health and human development neonatal research network. neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics. 2010;126:443–456. - PMC - PubMed
-
- Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, Wrage LA, Poole K. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. - PubMed
-
- Gough A, Spence D, Linden M, Halliday HL, McGarvey LP. General and respiratory health outcomes in adult survivors of bronchopulmonary dysplasia: a systematic review. Chest. 2012;141:1554–1567. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

