Human mobility and the spatial transmission of influenza in the United States
- PMID: 28187123
- PMCID: PMC5349690
- DOI: 10.1371/journal.pcbi.1005382
Human mobility and the spatial transmission of influenza in the United States
Abstract
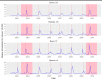
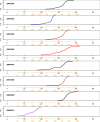
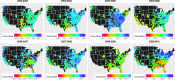
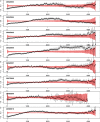
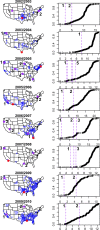
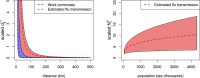
Seasonal influenza epidemics offer unique opportunities to study the invasion and re-invasion waves of a pathogen in a partially immune population. Detailed patterns of spread remain elusive, however, due to lack of granular disease data. Here we model high-volume city-level medical claims data and human mobility proxies to explore the drivers of influenza spread in the US during 2002-2010. Although the speed and pathways of spread varied across seasons, seven of eight epidemics likely originated in the Southern US. Each epidemic was associated with 1-5 early long-range transmission events, half of which sparked onward transmission. Gravity model estimates indicate a sharp decay in influenza transmission with the distance between infectious and susceptible cities, consistent with spread dominated by work commutes rather than air traffic. Two early-onset seasons associated with antigenic novelty had particularly localized modes of spread, suggesting that novel strains may spread in a more localized fashion than previously anticipated.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

