Neural mechanisms of rhythm-based temporal prediction: Delta phase-locking reflects temporal predictability but not rhythmic entrainment
- PMID: 28187128
- PMCID: PMC5302287
- DOI: 10.1371/journal.pbio.2001665
Neural mechanisms of rhythm-based temporal prediction: Delta phase-locking reflects temporal predictability but not rhythmic entrainment
Abstract
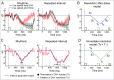
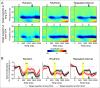
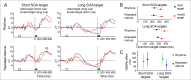
Predicting the timing of upcoming events enables efficient resource allocation and action preparation. Rhythmic streams, such as music, speech, and biological motion, constitute a pervasive source for temporal predictions. Widely accepted entrainment theories postulate that rhythm-based predictions are mediated by synchronizing low-frequency neural oscillations to the rhythm, as indicated by increased phase concentration (PC) of low-frequency neural activity for rhythmic compared to random streams. However, we show here that PC enhancement in scalp recordings is not specific to rhythms but is observed to the same extent in less periodic streams if they enable memory-based prediction. This is inconsistent with the predictions of a computational entrainment model of stronger PC for rhythmic streams. Anticipatory change in alpha activity and facilitation of electroencephalogram (EEG) manifestations of response selection are also comparable between rhythm- and memory-based predictions. However, rhythmic sequences uniquely result in obligatory depression of preparation-related premotor brain activity when an on-beat event is omitted, even when it is strategically beneficial to maintain preparation, leading to larger behavioral costs for violation of prediction. Thus, while our findings undermine the validity of PC as a sign of rhythmic entrainment, they constitute the first electrophysiological dissociation, to our knowledge, between mechanisms of rhythmic predictions and of memory-based predictions: the former obligatorily lead to resonance-like preparation patterns (that are in line with entrainment), while the latter allow flexible resource allocation in time regardless of periodicity in the input. Taken together, they delineate the neural mechanisms of three distinct modes of preparation: continuous vigilance, interval-timing-based prediction and rhythm-based prediction.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Comment in
-
Dance to the rhythm, cautiously: Isolating unique indicators of oscillatory entrainment.PLoS Biol. 2017 Sep 19;15(9):e2003534. doi: 10.1371/journal.pbio.2003534. eCollection 2017 Sep. PLoS Biol. 2017. PMID: 28926563 Free PMC article. No abstract available.
-
What do we talk about when we talk about rhythm?PLoS Biol. 2017 Sep 19;15(9):e2002794. doi: 10.1371/journal.pbio.2002794. eCollection 2017 Sep. PLoS Biol. 2017. PMID: 28926570 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

