Understanding the Intersections between Metabolism and Cancer Biology
- PMID: 28187287
- PMCID: PMC5329766
- DOI: 10.1016/j.cell.2016.12.039
Understanding the Intersections between Metabolism and Cancer Biology
Abstract
Transformed cells adapt metabolism to support tumor initiation and progression. Specific metabolic activities can participate directly in the process of transformation or support the biological processes that enable tumor growth. Exploiting cancer metabolism for clinical benefit requires defining the pathways that are limiting for cancer progression and understanding the context specificity of metabolic preferences and liabilities in malignant cells. Progress toward answering these questions is providing new insight into cancer biology and can guide the more effective targeting of metabolism to help patients.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

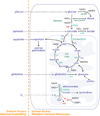
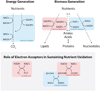

References
-
- Andronesi OC, Kim GS, Gerstner E, Batchelor T, Tzika AA, Fantin VR, Vander Heiden MG, Sorensen AG. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Science translational medicine. 2012;4:116ra114. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

