Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial
- PMID: 28187400
- PMCID: PMC6080717
- DOI: 10.1016/j.psyneuen.2017.01.023
Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial
Abstract
Objective: Currently, no valid measures inform treatment selection for depressed patients. Whether C-reactive protein (CRP) in particular and two other acute phase reactants (inflammatory markers) could differentiate between patients responding to either of two treatments with different mechanisms of action was assessed.
Method: Subjects included Combining Medications to Enhance Depression Outcomes (CO-MED) trial participants randomly assigned to either escitalopram plus placebo (SSRI monotherapy, n=51) or bupropion plus escitalopram combination (bupropion-SSRI combination, n=55) with baseline plasma samples. CRP, serum amyloid P component, and alpha-2-macroglobulin were measured using the Bioplex Pro™ human acute-phase 4-plex panel. We conducted mixed model analyses of depressive symptom (Quick Inventory of Depressive Symptomatology Self-Report) and side-effect burden (Frequency, Intensity, and Burden of Side-Effects Rating Scale) obtained weekly or every other week over the 12-week acute-phase of CO-MED trial to evaluate the relationship between these outcomes and baseline CRP and other acute-phase reactants.
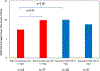
Results: The treatment arms did not differ in depressive symptom or side effect outcomes. Most participants (69.8%, 74/106) had baseline CRP levels greater than 1mg/L (indicative of systemic inflammatory activity). Higher baseline CRP levels were associated lower depression severity (correlation coefficient=-0.63) with bupropion-SSRI combination but not with SSRI monotherapy (correlation coefficient=0.40). The overall remission rate was 41.5%. The estimated remission rate with CRP threshold based assignment (SSRI monotherapy for <1mg/L and Bupropion-SSRI for ≥1mg/L) was 53.1%, with a number needed to treat of 8.6. Side effect burden was unrelated to any baseline inflammatory marker.
Conclusions: Baseline CRP levels relate differentially to antidepressant treatment outcomes in persons with major depressive disorder. Clinicaltrials.gov identifier: NCT00590863.
Keywords: Antidepressant response; Biomarker; C-reactive protein; Depression; Inflammation.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures

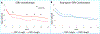

Similar articles
-
Interleukin 17 selectively predicts better outcomes with bupropion-SSRI combination: Novel T cell biomarker for antidepressant medication selection.Brain Behav Immun. 2017 Nov;66:103-110. doi: 10.1016/j.bbi.2017.07.005. Epub 2017 Jul 8. Brain Behav Immun. 2017. PMID: 28698115 Free PMC article.
-
Baseline depression severity as a predictor of single and combination antidepressant treatment outcome: results from the CO-MED trial.Eur Neuropsychopharmacol. 2012 Mar;22(3):183-99. doi: 10.1016/j.euroneuro.2011.07.010. Epub 2011 Sep 14. Eur Neuropsychopharmacol. 2012. PMID: 21920711 Free PMC article. Clinical Trial.
-
The impact of chronic depression on acute and long-term outcomes in a randomized trial comparing selective serotonin reuptake inhibitor monotherapy versus each of 2 different antidepressant medication combinations.J Clin Psychiatry. 2012 Jul;73(7):967-76. doi: 10.4088/JCP.11m07043. Epub 2012 May 29. J Clin Psychiatry. 2012. PMID: 22687487 Clinical Trial.
-
Novel Augmentation Strategies in Major Depression.Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review.
-
Pharmacological Monotherapy for Depressive Disorders: Current and Future-A Narrative Review.Medicina (Kaunas). 2025 Mar 21;61(4):558. doi: 10.3390/medicina61040558. Medicina (Kaunas). 2025. PMID: 40282849 Free PMC article. Review.
Cited by
-
Neuroimaging Advances for Depression.Cerebrum. 2017 Nov 1;2017:cer-16-17. eCollection 2017 Nov-Dec. Cerebrum. 2017. PMID: 30210664 Free PMC article.
-
Serum High-Sensitivity C-Reactive Protein Is Associated with Postoperative Psychiatric Status in Patients with Empty Nose Syndrome.Diagnostics (Basel). 2021 Dec 18;11(12):2388. doi: 10.3390/diagnostics11122388. Diagnostics (Basel). 2021. PMID: 34943627 Free PMC article.
-
Major Depressive Disorder, Inflammation, and Nutrition: A Tricky Pattern?Nutrients. 2023 Aug 3;15(15):3438. doi: 10.3390/nu15153438. Nutrients. 2023. PMID: 37571376 Free PMC article.
-
New lung cancer treatments (immunotherapy and targeted therapies) and their associations with depression and other psychological side effects as compared to chemotherapy.Gen Hosp Psychiatry. 2019 Sep-Oct;60:148-155. doi: 10.1016/j.genhosppsych.2019.04.001. Epub 2019 Apr 18. Gen Hosp Psychiatry. 2019. PMID: 31056371 Free PMC article.
-
Higher levels of plasma inflammation biomarkers are associated with depressed mood and quality of life in aging, virally suppressed men, but not women, with HIV.Brain Behav Immun Health. 2020 Aug 13;7:100121. doi: 10.1016/j.bbih.2020.100121. eCollection 2020 Aug. Brain Behav Immun Health. 2020. PMID: 34589877 Free PMC article.
References
-
- Arnow BA, Blasey C, Williams LM, Palmer DM, Rekshan W, Schatzberg AF, Etkin A, Kulkarni J, Luther JF, Rush AJ, 2015. Depression Subtypes in Predicting Antidepressant Response: A Report From the iSPOT-D Trial. Am J Psychiatry 172, 743–750. - PubMed
-
- Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, Golden RN, Martin P, Potter WZ, Richelson E, et al., 1995. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry 56, 395–401. - PubMed
-
- Brustolim D, Ribeiro-dos-Santos R, Kast RE, Altschuler EL, Soares MB, 2006. A new chapter opens in anti-inflammatory treatments: the antidepressant bupropion lowers production of tumor necrosis factor-alpha and interferon-gamma in mice. Int Immunopharmacol 6, 903–907. - PubMed
-
- Cremers TI, Flik G, Folgering JH, Rollema H, Stratford RE Jr., 2016. Development of a Rat Plasma and Brain Extracellular Fluid Pharmacokinetic Model for Bupropion and Hydroxybupropion Based on Microdialysis Sampling, and Application to Predict Human Brain Concentrations. Drug Metab Dispos 44, 624–633. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

