Interface between breast cancer cells and the tumor microenvironment using platelet-rich plasma to promote tumor angiogenesis - influence of platelets and fibrin bundles on the behavior of breast tumor cells
- PMID: 28187434
- PMCID: PMC5370006
- DOI: 10.18632/oncotarget.15170
Interface between breast cancer cells and the tumor microenvironment using platelet-rich plasma to promote tumor angiogenesis - influence of platelets and fibrin bundles on the behavior of breast tumor cells
Abstract
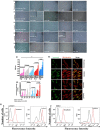
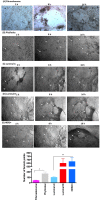
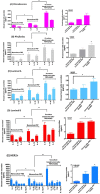
Cancer progression is associated with an evolving tissue interface of direct epithelial-tumor microenvironment interactions. In biopsies of human breast tumors, extensive alterations in molecular pathways are correlated with cancer staging on both sides of the tumor-stroma interface. These interactions provide a pivotal paracrine signaling to induce malignant phenotype transition, the epithelial-mesenchymal transition (EMT). We explored how the direct contact between platelets-fibrin bundles primes metastasis using platelet-rich plasma (PRP) as a source of growth factors and mimics the provisional fibrin matrix between actively growing breast cancer cells and the tumor stroma. We have demonstrated PRP functions, modulating cell proliferation that is tumor-subtype and cancer cell-type-specific. Epithelial and stromal primary cells were prepared from breast cancer biopsies from 21 women with different cancer subtypes. Cells supplemented with PRP were immunoblotted with anti-phospho and total Src-Tyr-416, FAK-Try-925, E-cadherin, N-cadherin, TGF-β, Smad2, and Snail monoclonal antibodies. Breast tumor cells from luminal B and HER2 subtypes showed the most malignant profiles and the expression of thrombin and other classes of proteases at levels that were detectable through FRET peptide libraries. The angiogenesis process was investigated in the interface obtained between platelet-fibrin-breast tumor cells co-cultured with HUVEC cells. Luminal B and HER2 cells showed robust endothelial cell capillary-like tubes ex vivo. The studied interface contributes to the attachment of endothelial cells, provides a source of growth factors, and is a solid substrate. Thus, replacement of FBS supplementation with PRP supplementation represents an efficient and simple approach for mimicking the real multifactorial tumor microenvironment.
Keywords: breast cancer; cancer; platelet-rich plasma; platelets; tumor microenvironment.
Conflict of interest statement
The authors declare that they have no conflicts of interest to disclose.
Figures




Similar articles
-
Malignant stroma increases luminal breast cancer cell proliferation and angiogenesis through platelet-derived growth factor signaling.BMC Cancer. 2014 Oct 1;14:735. doi: 10.1186/1471-2407-14-735. BMC Cancer. 2014. PMID: 25274034 Free PMC article.
-
Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro.Clin Orthop Relat Res. 2015 May;473(5):1635-43. doi: 10.1007/s11999-015-4192-2. Clin Orthop Relat Res. 2015. PMID: 25690170 Free PMC article.
-
Cathepsin K induces platelet dysfunction and affects cell signaling in breast cancer - molecularly distinct behavior of cathepsin K in breast cancer.BMC Cancer. 2016 Mar 1;16:173. doi: 10.1186/s12885-016-2203-7. BMC Cancer. 2016. PMID: 26931461 Free PMC article.
-
Microbicidal properties of Leukocyte- and Platelet-Rich Plasma/Fibrin (L-PRP/L-PRF): new perspectives.J Biol Regul Homeost Agents. 2012 Apr-Jun;26(2 Suppl 1):43S-52S. J Biol Regul Homeost Agents. 2012. PMID: 23648198 Review.
-
Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF).Trends Biotechnol. 2009 Mar;27(3):158-67. doi: 10.1016/j.tibtech.2008.11.009. Epub 2009 Jan 31. Trends Biotechnol. 2009. PMID: 19187989 Review.
Cited by
-
Reviewing cancer's biology: an eclectic approach.J Egypt Natl Canc Inst. 2021 Nov 1;33(1):32. doi: 10.1186/s43046-021-00088-y. J Egypt Natl Canc Inst. 2021. PMID: 34719756 Review.
-
Indications and contraindications to platelet-rich plasma injections in musculoskeletal diseases in case of infectious, oncological and haematological comorbidities: A 2025 formal consensus from the GRIIP (International Research Group on Platelet Injections).Knee Surg Sports Traumatol Arthrosc. 2025 Jun;33(6):2293-2306. doi: 10.1002/ksa.12682. Epub 2025 Apr 22. Knee Surg Sports Traumatol Arthrosc. 2025. PMID: 40260684 Free PMC article.
-
Non-Muscular Invasive Bladder Cancer: Re-envisioning Therapeutic Journey from Traditional to Regenerative Interventions.Aging Dis. 2021 Jun 1;12(3):868-885. doi: 10.14336/AD.2020.1109. eCollection 2021 Jun. Aging Dis. 2021. PMID: 34094648 Free PMC article. Review.
-
Biotech-Educated Platelets: Beyond Tissue Regeneration 2.0.Int J Mol Sci. 2020 Aug 23;21(17):6061. doi: 10.3390/ijms21176061. Int J Mol Sci. 2020. PMID: 32842455 Free PMC article. Review.
-
In Vivo Observation of Endothelial Cell-Assisted Vascularization in Pancreatic Cancer Xenograft Engineering.Tissue Eng Regen Med. 2018 Feb 3;15(3):275-285. doi: 10.1007/s13770-018-0113-2. eCollection 2018 Jun. Tissue Eng Regen Med. 2018. PMID: 30603553 Free PMC article.
References
-
- Kuznetsov HS, Marsh T, Markens BA, Castaño Z, Greene-Colozzi A, Hay SA, Brown VE, Richardson AL, Signoretti S, Battinelli EM, McAllister SS. Identification of luminal breast cancers that establish a tumor-supportive macroenvironment defined by proangiogenic platelets and bone marrow-derived cell. Cancer Discov. 2012;2:1150–65. - PMC - PubMed
-
- Scheel C, Onder T, Karnoub A, Weinberg RA. Adaptation versus selection: the origins of metastatic behavior. Cancer Res. 2007;2:11476–9. - PubMed
-
- Angelucci C, Maulucci G, Lama G, Proietti G, Colabianchi A, Papi M, Maiorana A, De Spirito M, Micera A, Balzamino OB, Di Leone A, Masetti R, Sica G. Epithelial-stromal interactions in human breast cancer: effects on adhesion, plasma membrane fluidity and migration speed and directness. PLoS One. 2012;7:e50804. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

