GMP-conformant on-site manufacturing of a CD133+ stem cell product for cardiovascular regeneration
- PMID: 28187777
- PMCID: PMC5303262
- DOI: 10.1186/s13287-016-0467-0
GMP-conformant on-site manufacturing of a CD133+ stem cell product for cardiovascular regeneration
Abstract
Background: CD133+ stem cells represent a promising subpopulation for innovative cell-based therapies in cardiovascular regeneration. Several clinical trials have shown remarkable beneficial effects following their intramyocardial transplantation. Yet, the purification of CD133+ stem cells is typically performed in centralized clean room facilities using semi-automatic manufacturing processes based on magnetic cell sorting (MACS®). However, this requires time-consuming and cost-intensive logistics.
Methods: CD133+ stem cells were purified from patient-derived sternal bone marrow using the recently developed automatic CliniMACS Prodigy® BM-133 System (Prodigy). The entire manufacturing process, as well as the subsequent quality control of the final cell product (CP), were realized on-site and in compliance with EU guidelines for Good Manufacturing Practice. The biological activity of automatically isolated CD133+ cells was evaluated and compared to manually isolated CD133+ cells via functional assays as well as immunofluorescence microscopy. In addition, the regenerative potential of purified stem cells was assessed 3 weeks after transplantation in immunodeficient mice which had been subjected to experimental myocardial infarction.
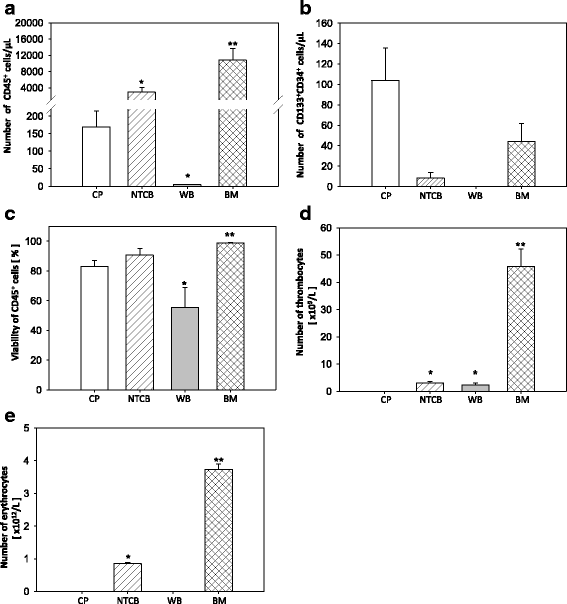
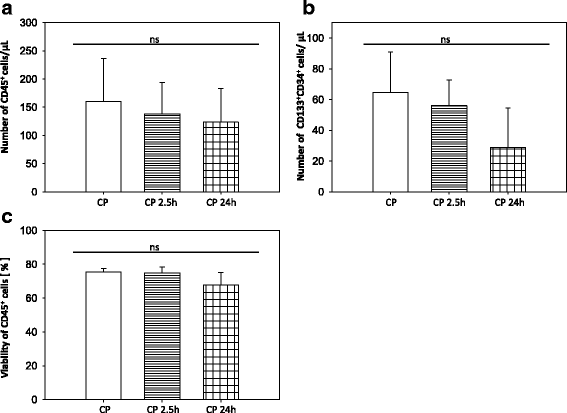
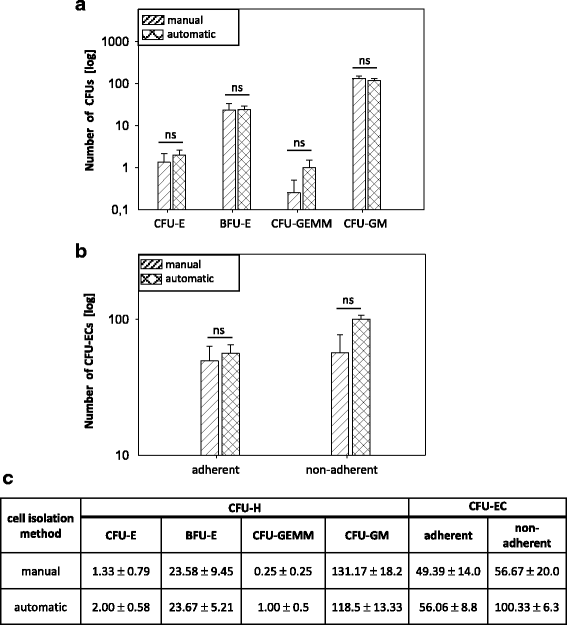
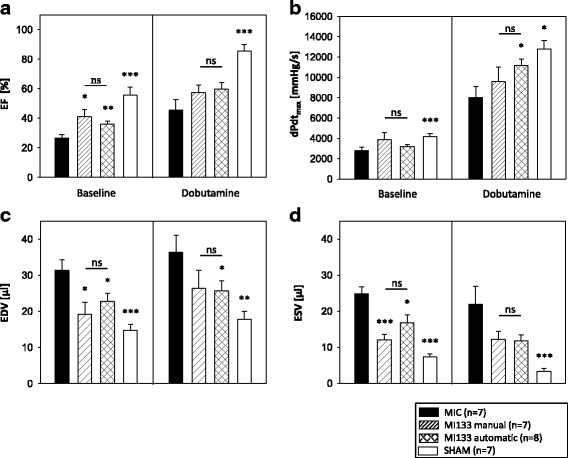
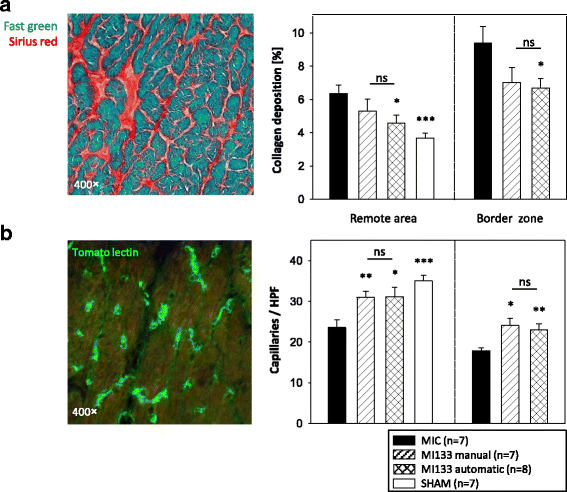
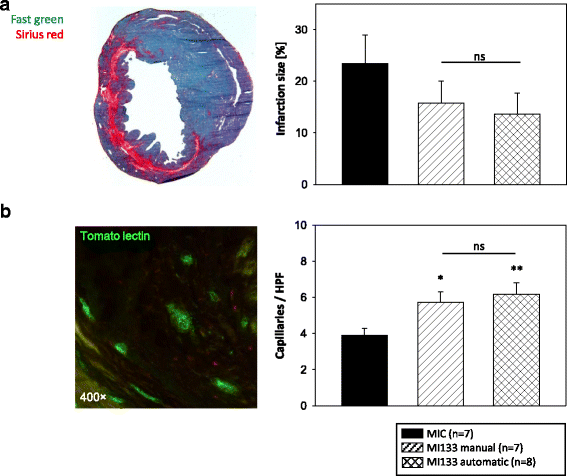
Results: We established for the first time an on-site manufacturing procedure for stem CPs intended for the treatment of ischemic heart diseases using an automatized system. On average, 0.88 × 106 viable CD133+ cells with a mean log10 depletion of 3.23 ± 0.19 of non-target cells were isolated. Furthermore, we demonstrated that these automatically isolated cells bear proliferation and differentiation capacities comparable to manually isolated cells in vitro. Moreover, the automatically generated CP shows equal cardiac regeneration potential in vivo.
Conclusions: Our results indicate that the Prodigy is a powerful system for automatic manufacturing of a CD133+ CP within few hours. Compared to conventional manufacturing processes, future clinical application of this system offers multiple benefits including stable CP quality and on-site purification under reduced clean room requirements. This will allow saving of time, reduced logistics and diminished costs.
Keywords: Adult hematopoietic stem cells; Advanced therapy medicinal product (ATMP); CD133+ cells; Cardiovascular regeneration; Clinical translation; Good Manufacturing Practice (GMP); Prodigy; Stem cell transplantation.
Figures
Similar articles
-
Angiogenic Potential of Bone Marrow Derived CD133+ and CD271+ Intramyocardial Stem Cell Trans- Plantation Post MI.Cells. 2019 Dec 27;9(1):78. doi: 10.3390/cells9010078. Cells. 2019. PMID: 31892273 Free PMC article.
-
Automation of cellular therapy product manufacturing: results of a split validation comparing CD34 selection of peripheral blood stem cell apheresis product with a semi-manual vs. an automatic procedure.J Transl Med. 2016 Mar 16;14:76. doi: 10.1186/s12967-016-0826-8. J Transl Med. 2016. PMID: 26983643 Free PMC article.
-
Automated CD34+ cell isolation of peripheral blood stem cell apheresis product.Cytotherapy. 2015 Oct;17(10):1465-71. doi: 10.1016/j.jcyt.2015.04.005. Epub 2015 May 14. Cytotherapy. 2015. PMID: 25981397
-
CD133(+) cells isolated from various sources and their role in future clinical perspectives.Expert Opin Biol Ther. 2010 Nov;10(11):1521-8. doi: 10.1517/14712598.2010.528386. Epub 2010 Oct 11. Expert Opin Biol Ther. 2010. PMID: 20932225 Review.
-
Adult BM stem cells regenerate mouse myocardium.Cytotherapy. 2002;4(6):521-5. doi: 10.1080/146532402761624674. Cytotherapy. 2002. PMID: 12568987 Review. No abstract available.
Cited by
-
CD271+ Human Mesenchymal Stem Cells Show Antiarrhythmic Effects in a Novel Murine Infarction Model.Cells. 2019 Nov 20;8(12):1474. doi: 10.3390/cells8121474. Cells. 2019. PMID: 31757119 Free PMC article.
-
CAR-T cell manufacturing landscape-Lessons from the past decade and considerations for early clinical development.Mol Ther Methods Clin Dev. 2024 Apr 16;32(2):101250. doi: 10.1016/j.omtm.2024.101250. eCollection 2024 Jun 13. Mol Ther Methods Clin Dev. 2024. PMID: 38737799 Free PMC article. Review.
-
Recent Progress in Stem Cell Modification for Cardiac Regeneration.Stem Cells Int. 2018 Jan 16;2018:1909346. doi: 10.1155/2018/1909346. eCollection 2018. Stem Cells Int. 2018. PMID: 29535769 Free PMC article. Review.
-
Data on the fate of MACS® MicroBeads intramyocardially co-injected with stem cell products.Data Brief. 2017 Jun 24;13:569-574. doi: 10.1016/j.dib.2017.06.035. eCollection 2017 Aug. Data Brief. 2017. PMID: 28706966 Free PMC article.
-
Cell Therapy in the Treatment of Coronary Heart Disease.Int J Mol Sci. 2023 Nov 28;24(23):16844. doi: 10.3390/ijms242316844. Int J Mol Sci. 2023. PMID: 38069167 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

