Cannabinoids Activate Monoaminergic Signaling to Modulate Key C. elegans Behaviors
- PMID: 28188220
- PMCID: PMC5354331
- DOI: 10.1523/JNEUROSCI.3151-16.2017
Cannabinoids Activate Monoaminergic Signaling to Modulate Key C. elegans Behaviors
Abstract
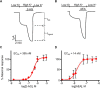
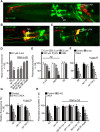
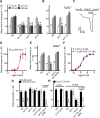
Cannabis sativa, or marijuana, a popular recreational drug, alters sensory perception and exerts a range of potential medicinal benefits. The present study demonstrates that the endogenous cannabinoid receptor agonists 2-arachidonoylglycerol (2-AG) and anandamide (AEA) activate a canonical cannabinoid receptor in Caenorhabditis elegans and also modulate monoaminergic signaling at multiple levels. 2-AG or AEA inhibit nociception and feeding through a pathway requiring the cannabinoid-like receptor NPR-19. 2-AG or AEA activate NPR-19 directly and cannabinoid-dependent inhibition can be rescued in npr-19-null animals by the expression of a human cannabinoid receptor, CB1, highlighting the orthology of the receptors. Cannabinoids also modulate nociception and locomotion through an NPR-19-independent pathway requiring an α2A-adrenergic-like octopamine (OA) receptor, OCTR-1, and a 5-HT1A-like serotonin (5-HT) receptor, SER-4, that involves a complex interaction among cannabinoid, octopaminergic, and serotonergic signaling. 2-AG activates OCTR-1 directly. In contrast, 2-AG does not activate SER-4 directly, but appears to enhance SER-4-dependent serotonergic signaling by increasing endogenous 5-HT. This study defines a conserved cannabinoid signaling system in C. elegans, demonstrates the cannabinoid-dependent activation of monoaminergic signaling, and highlights the advantages of studying cannabinoid signaling in a genetically tractable whole-animal model.SIGNIFICANCE STATEMENTCannabis sativa, or marijuana, causes euphoria and exerts a wide range of medicinal benefits. For years, cannabinoids have been studied at the cellular level using tissue explants with conflicting results. To better understand cannabinoid signaling, we have used the Caenorhabditis elegans model to examine the effects of cannabinoids on behavior. The present study demonstrates that mammalian cannabinoid receptor ligands activate a conserved cannabinoid signaling system in C. elegans and also modulate monoaminergic signaling, potentially affecting an array of disorders, including anxiety and depression. This study highlights the potential role of cannabinoids in modulating monoaminergic signaling and the advantages of studying cannabinoid signaling in a genetically tractable, whole-animal model.
Keywords: C. elegans; cannabinoid; monoamine; neuromodulation; nociception.
Copyright © 2017 the authors 0270-6474/17/372859-11$15.00/0.
Figures



Similar articles
-
Cannabinoids Stimulate the TRP Channel-Dependent Release of Both Serotonin and Dopamine to Modulate Behavior in C. elegans.J Neurosci. 2019 May 22;39(21):4142-4152. doi: 10.1523/JNEUROSCI.2371-18.2019. Epub 2019 Mar 18. J Neurosci. 2019. PMID: 30886012 Free PMC article.
-
Opiates Modulate Noxious Chemical Nociception through a Complex Monoaminergic/Peptidergic Cascade.J Neurosci. 2016 May 18;36(20):5498-508. doi: 10.1523/JNEUROSCI.4520-15.2016. J Neurosci. 2016. PMID: 27194330 Free PMC article.
-
The monoaminergic modulation of sensory-mediated aversive responses in Caenorhabditis elegans requires glutamatergic/peptidergic cotransmission.J Neurosci. 2010 Jun 9;30(23):7889-99. doi: 10.1523/JNEUROSCI.0497-10.2010. J Neurosci. 2010. PMID: 20534837 Free PMC article.
-
The Endocannabinoid System Modulating Levels of Consciousness, Emotions and Likely Dream Contents.CNS Neurol Disord Drug Targets. 2017;16(4):370-379. doi: 10.2174/1871527316666170223161908. CNS Neurol Disord Drug Targets. 2017. PMID: 28240187 Review.
-
The neurobiology and evolution of cannabinoid signalling.Philos Trans R Soc Lond B Biol Sci. 2001 Mar 29;356(1407):381-408. doi: 10.1098/rstb.2000.0787. Philos Trans R Soc Lond B Biol Sci. 2001. PMID: 11316486 Free PMC article. Review.
Cited by
-
Cannabinoid-induced lower lip retraction in rats.Psychopharmacology (Berl). 2019 Apr;236(4):1199-1206. doi: 10.1007/s00213-018-5125-z. Epub 2018 Nov 20. Psychopharmacology (Berl). 2019. PMID: 30460515 Free PMC article.
-
The Two Faces of Nematode Infection: Virulence and Immunomodulatory Molecules From Nematode Parasites of Mammals, Insects and Plants.Front Microbiol. 2020 Dec 2;11:577846. doi: 10.3389/fmicb.2020.577846. eCollection 2020. Front Microbiol. 2020. PMID: 33343521 Free PMC article. Review.
-
Esculetin Inhibits Fat Accumulation Through Insulin/Insulin-like Growth Factor- and AMP-Activated Protein Kinase-Dependent Pathways in Caenorhabditis elegans.Nutrients. 2025 May 1;17(9):1565. doi: 10.3390/nu17091565. Nutrients. 2025. PMID: 40362874 Free PMC article.
-
Juniperonic Acid Biosynthesis is Essential in Caenorhabditis Elegans Lacking Δ6 Desaturase (fat-3) and Generates New ω-3 Endocannabinoids.Cells. 2020 Sep 19;9(9):2127. doi: 10.3390/cells9092127. Cells. 2020. PMID: 32961767 Free PMC article.
-
Cannabinoids and healthy ageing: the potential for extending healthspan and lifespan in preclinical models with an emphasis on Caenorhabditis elegans.Geroscience. 2024 Dec;46(6):5643-5661. doi: 10.1007/s11357-024-01162-8. Epub 2024 May 2. Geroscience. 2024. PMID: 38696056 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
