Lung pericyte-like cells are functional interstitial immune sentinel cells
- PMID: 28188224
- PMCID: PMC5407093
- DOI: 10.1152/ajplung.00349.2016
Lung pericyte-like cells are functional interstitial immune sentinel cells
Abstract
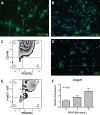
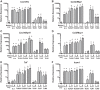
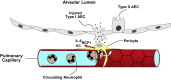
Pericytes are perivascular PDGF receptor-β+ (PDGFRβ+) stromal cells required for vasculogenesis and maintenance of microvascular homeostasis in many organs. Because of their unique juxtaposition to microvascular endothelium, lung PDGFRβ+ cells are well situated to detect proinflammatory molecules released following epithelial injury and promote acute inflammatory responses. Thus we hypothesized that these cells represent an unrecognized immune surveillance or injury-sentinel interstitial cell. To evaluate this hypothesis, we isolated PDGFRβ+ cells from murine lung and demonstrated that they have characteristics consistent with a pericyte population (referred to as pericyte-like cells for simplicity hereafter). We showed that pericyte-like cells expressed functional Toll-like receptors and upregulated chemokine expression following exposure to bronchoalveolar lavage fluid (BALF) collected from mice with sterile lung injury. Interestingly, BALF from mice without lung injury also induced chemokine expression in pericyte-like cells, suggesting that pericyte-like cells are primed to sense epithelial injury (permeability changes). Following LPS-induced lung inflammation, increased numbers of pericyte-like cells expressed IL-6, chemokine (C-X-C motif) ligand-1, chemokine (C-C motif) ligand 2/ monocyte chemotactic protein-1, and ICAM-1 in vivo. Sterile lung injury in pericyte-ablated mice was associated with decreased inflammation compared with normal mice. In summary, we found that pericyte-like cells are immune responsive and express diverse chemokines in response to lung injury in vitro and in vivo. Furthermore, pericyte-like cell ablation attenuated inflammation in sterile lung injury, suggesting that these cells play an important functional role in mediating lung inflammatory responses. We propose a model in which pericyte-like cells function as interstitial immune sentinels, detecting proinflammatory molecules released following epithelial barrier damage and participating in recruitment of circulating leukocytes.
Keywords: acute lung injury; damage-associated molecular patterns; inflammation; innate immunity; pericytes.
Copyright © 2017 the American Physiological Society.
Figures


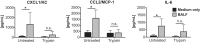


References
-
- Covas DT, Panepucci RA, Fontes AM, Silva WA Jr, Orellana MD, Freitas MCC, Neder L, Santos ARD, Peres LC, Jamur MC, Zago MA. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol 36: 642–654, 2008. doi: 10.1016/j.exphem.2007.12.015. - DOI - PubMed
-
- Guijarro-Muñoz I, Compte M, Álvarez-Cienfuegos A, Álvarez-Vallina L, Sanz L. Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated NF-κB signaling pathway and proinflammatory response in human pericytes. J Biol Chem 289: 2457–2468, 2014. doi: 10.1074/jbc.M113.521161. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

