Human Keratinocytes Respond to Extracellular UTP by Induction of Hyaluronan Synthase 2 Expression and Increased Hyaluronan Synthesis
- PMID: 28188289
- PMCID: PMC5377801
- DOI: 10.1074/jbc.M116.760322
Human Keratinocytes Respond to Extracellular UTP by Induction of Hyaluronan Synthase 2 Expression and Increased Hyaluronan Synthesis
Abstract
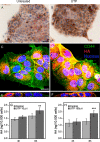
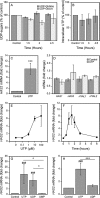
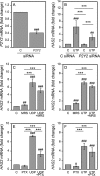
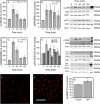
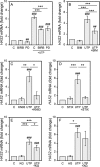
The release of nucleotides into extracellular space is triggered by insults like wounding and ultraviolet radiation, resulting in stimulatory or inhibitory signals via plasma membrane nucleotide receptors. As similar insults are known to activate hyaluronan synthesis we explored the possibility that extracellular UTP or its breakdown products UDP and UMP act as mediators for hyaluronan synthase (HAS) activation in human epidermal keratinocytes. UTP increased hyaluronan both in the pericellular matrix and in the culture medium of HaCaT cells. 10-100 μm UTP strongly up-regulated HAS2 expression, although the other hyaluronan synthases (HAS1, HAS3) and hyaluronidases (HYAL1, HYAL2) were not affected. The HAS2 response was rapid and transient, with the maximum stimulation at 1.5 h. UDP exerted a similar effect, but higher concentrations were required for the response, and UMP showed no stimulation at all. Specific siRNAs against the UTP receptor P2Y2, and inhibitors of UDP receptors P2Y6 and P2Y14, indicated that the response to UTP was mediated mainly through P2Y2 and to a lesser extent via UDP receptors. UTP increased the phosphorylation of p38, ERK, CREB, and Ser-727 of STAT3 and induced nuclear translocation of pCaMKII. Inhibitors of PKC, p38, ERK, CaMKII, STAT3, and CREB partially blocked the activation of HAS2 expression, confirming the involvement of these pathways in the UTP-induced HAS2 response. The present data reveal a selective up-regulation of HAS2 expression by extracellular UTP, which is likely to contribute to the previously reported rapid activation of hyaluronan metabolism in response to tissue trauma or ultraviolet radiation.
Keywords: P2Y; UTP; cell signaling; extracellular nucleotide; hyaluronan; hyaluronan synthesis; keratinocyte; nucleotide; purinergic receptor.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

Comment in
-
Matrix Biology Highlights.Matrix Biol. 2017 May;59:1-2. doi: 10.1016/j.matbio.2017.03.001. Matrix Biol. 2017. PMID: 28335831 No abstract available.
References
-
- Inoue K., Hosoi J., and Denda M. (2007) Extracellular ATP has stimulatory effects on the expression and release of IL-6 via purinergic receptors in normal human epidermal keratinocytes. J. Invest. Dermatol. 127, 362–371 - PubMed
-
- Pastore S., Mascia F., Gulinelli S., Forchap S., Dattilo C., Adinolfi E., Girolomoni G., Di Virgilio F., and Ferrari D. (2007) Stimulation of purinergic receptors modulates chemokine expression in human keratinocytes. J. Invest. Dermatol. 127, 660–667 - PubMed
-
- Holzer A. M., and Granstein R. D. (2004) Role of extracellular adenosine triphosphate in human skin. J. Cutan. Med. Surg. 8, 90–96 - PubMed
-
- Azorin N., Raoux M., Rodat-Despoix L., Merrot T., Delmas P., and Crest M. (2011) ATP signalling is crucial for the response of human keratinocytes to mechanical stimulation by hypo-osmotic shock. Exp. Dermatol. 20, 401–407 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

