Negative Stain Single-particle EM of the Maltose Transporter in Nanodiscs Reveals Asymmetric Closure of MalK2 and Catalytic Roles of ATP, MalE, and Maltose
- PMID: 28188291
- PMCID: PMC5392688
- DOI: 10.1074/jbc.M116.757898
Negative Stain Single-particle EM of the Maltose Transporter in Nanodiscs Reveals Asymmetric Closure of MalK2 and Catalytic Roles of ATP, MalE, and Maltose
Abstract
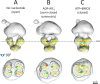
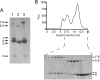
The Escherichia coli MalE-MalFGK2 complex is one of the best characterized members of the large and ubiquitous family of ATP-binding cassette (ABC) transporters. It is composed of a membrane-spanning heterodimer, MalF-MalG; a homodimeric ATPase, MalK2; and a periplasmic maltose receptor, MalE. Opening and closure of MalK2 is coupled to conformational changes in MalF-MalG and the alternate exposition of the substrate-binding site to either side of the membrane. To further define this alternate access mechanism and the impact of ATP, MalE, and maltose on the conformation of the transporter during the transport cycle, we have reconstituted MalFGK2 in nanodiscs and analyzed its conformations under 10 different biochemical conditions using negative stain single-particle EM. EM map results (at 15-25 Å resolution) indicate that binding of ATP to MalK2 promotes an asymmetric, semi-closed conformation in accordance with the low ATPase activity of MalFGK2 In the presence of MalE, the MalK dimer becomes fully closed, gaining the ability to hydrolyze ATP. In the presence of ADP or maltose, MalE·MalFGK2 remains essentially in a semi-closed symmetric conformation, indicating that release of these ligands is required for the return to the initial state. Taken together, this structural information provides a rationale for the stimulation of MalK ATPase activity by MalE as well as by maltose.
Keywords: ABC transporter; electron microscopy (EM); membrane protein; membrane transporter reconstitution; single-particle analysis; translocation; transport.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




References
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

