Asymmetric configurations in a reengineered homodimer reveal multiple subunit communication pathways in protein allostery
- PMID: 28188293
- PMCID: PMC5391741
- DOI: 10.1074/jbc.M117.776047
Asymmetric configurations in a reengineered homodimer reveal multiple subunit communication pathways in protein allostery
Abstract
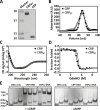
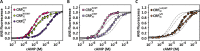
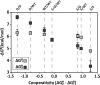
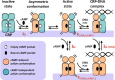
Many allosteric proteins form homo-oligomeric complexes to regulate a biological function. In homo-oligomers, subunits establish communication pathways that are modulated by external stimuli like ligand binding. A challenge for dissecting the communication mechanisms in homo-oligomers is identifying intermediate liganded states, which are typically transiently populated. However, their identities provide the most mechanistic information on how ligand-induced signals propagate from bound to empty subunits. Here, we dissected the directionality and magnitude of subunit communication in a reengineered single-chain version of the homodimeric transcription factor cAMP receptor protein. By combining wild-type and mutant subunits in various asymmetric configurations, we revealed a linear relationship between the magnitude of cooperative effects and the number of mutant subunits. We found that a single mutation is sufficient to change the global allosteric behavior of the dimer even when one subunit was wild type. Dimers harboring two mutations with opposite cooperative effects had different allosteric properties depending on the arrangement of the mutations. When the two mutations were placed in the same subunit, the resulting cooperativity was neutral. In contrast, when placed in different subunits, the observed cooperativity was dominated by the mutation with strongest effects over cAMP affinity relative to wild type. These results highlight the distinct roles of intrasubunit interactions and intersubunit communication in allostery. Finally, dimers bound to either one or two cAMP molecules had similar DNA affinities, indicating that both asymmetric and symmetric liganded states activate DNA interactions. These studies have revealed the multiple communication pathways that homo-oligomers employ to transduce signals.
Keywords: allosteric regulation; cAMP receptor protein (CRP); conformational change; cooperativity; cyclic AMP (cAMP); single-chain dimer; subunit communication; transcription factor.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
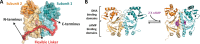


References
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

