Effect of Extending the Original Eligibility Criteria for the CROSS Neoadjuvant Chemoradiotherapy on Toxicity and Survival in Esophageal Cancer
- PMID: 28188501
- PMCID: PMC5486922
- DOI: 10.1245/s10434-017-5797-3
Effect of Extending the Original Eligibility Criteria for the CROSS Neoadjuvant Chemoradiotherapy on Toxicity and Survival in Esophageal Cancer
Abstract
Background: Patients with curable esophageal cancer (EC) who proceed beyond the original Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study (CROSS) eligibility criteria are also treated with neoadjuvant chemoradiotherapy (nCRT). This study assessed the effect that extending the CROSS eligibility criteria for nCRT has on treatment-related toxicity and overall survival (OS) in EC.
Methods: The study enrolled 161 patients with locally advanced EC (T1N1-3/T2-4aN0-3/M0) treated with the CROSS schedule followed by esophagectomy. Group 1 consisted of 89 patients who met the CROSS criteria, and group 2 consisted of 72 patients who met the extended eligibility criteria, i.e. a tumor length greater than 8 cm (n = 24), more than 10% weight loss (n = 35), more than 2-4 cm extension in the stomach (n = 21), celiac lymph node metastasis (n = 13), and/or age over 75 years (n = 2). The study assessed the differences in nCRT-associated toxicity [National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) grade ≥ 3] and 90-day postoperative mortality. Moreover, the prognostic value for OS was assessed with multivariate Cox regression analysis.
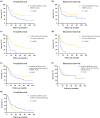
Results: No difference was found in nCRT-associated toxicity (P = 0.117), postoperative complications (P = 0.783), and 90-day mortality (P = 0.492). The OS differed significantly (P = 0.004), with a median of 37.3 months [95% confidence interval (CI), 10.4-64.2 months] for group 1 and 17.2 months (95% CI 13.8-20.7 months) for group 2. Pathologic N stage (P = 0.023), pathologic T stage (P = 0.043), and group 2 (P = 0.008) were independent prognostic factors for OS.
Conclusions: Extension of the CROSS study eligibility criteria for nCRT did not affect nCRT-associated toxicity, postoperative complications, and postoperative mortality, but was prognostic for OS.
Conflict of interest statement
The authors have nothing to disclose.
Figures
References
-
- American Joint Committee on Cancer . AJCC Cancer Staging Manual. 6. New York: Springer; 2002.
-
- American Joint Committee on Cancer . AJCC Cancer Staging Manual. 7. New York: Springer; 2009.
-
- Schrauwen RWM, Bisseling TM, Bonenkamp JJ, Radema SA, Rütten H, Rosman C, et al. Uitkomsten van neoadjuvante chemoradiotherapie gevolgd door slokdarmresectie bij patiënten met een hogere leeftijd of een grotere tumorlengte. NTvO. 2015;12:50–57.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

