Characterization of a novel intrinsically radiopaque Drug-eluting Bead for image-guided therapy: DC Bead LUMI™
- PMID: 28188808
- PMCID: PMC5416940
- DOI: 10.1016/j.jconrel.2017.02.001
Characterization of a novel intrinsically radiopaque Drug-eluting Bead for image-guided therapy: DC Bead LUMI™
Abstract
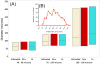
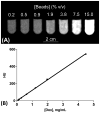
We have developed a straightforward and efficient method of introducing radiopacity into Polyvinyl alcohol (PVA)-2-Acrylamido-2-methylpropane sulfonic acid (AMPS) hydrogel beads (DC Bead™) that are currently used in the clinic to treat liver malignancies. Coupling of 2,3,5-triiodobenzaldehyde to the PVA backbone of pre-formed beads yields a uniformly distributed level of iodine attached throughout the bead structure (~150mg/mL) which is sufficient to be imaged under standard fluoroscopy and computed tomography (CT) imaging modalities used in treatment procedures (DC Bead LUMI™). Despite the chemical modification increasing the density of the beads to ~1.3g/cm3 and the compressive modulus by two orders of magnitude, they remain easily suspended, handled and administered through standard microcatheters. As the core chemistry of DC Bead LUMI™ is the same as DC Bead™, it interacts with drugs using ion-exchange between sulfonic acid groups on the polymer and the positively charged amine groups of the drugs. Both doxorubicin (Dox) and irinotecan (Iri) elution kinetics for all bead sizes evaluated were within the parameters already investigated within the clinic for DC Bead™. Drug loading did not affect the radiopacity and there was a direct relationship between bead attenuation and Dox concentration. The ability (Dox)-loaded DC Bead LUMI™ to be visualized in vivo was demonstrated by the administration of into hepatic arteries of a VX2 tumor-bearing rabbit under fluoroscopy, followed by subsequent CT imaging.
Keywords: Controlled drug release; Radiopaque Drug-eluting Beads; Transarterial chemoembolization; X-ray imaging.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures





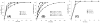
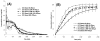
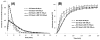

References
-
- Lewis AL. DC Bead(TM): a major development in the toolbox for the interventional oncologist. Expert Rev Med Devices. 2009;6:389–400. - PubMed
-
- Lewis AL, Gonzalez MV, Lloyd AW, Hall B, Tang Y, Willis SL, et al. DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol. 2006;17:335–42. - PubMed
-
- Nicolini A, Crespi S, Martinetti L. Drug delivery embolization systems: a physician’s perspective. Expert opinion on drug delivery. 2011;8:1071–84. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

