Co-clinical quantitative tumor volume imaging in ALK-rearranged NSCLC treated with crizotinib
- PMID: 28189201
- PMCID: PMC5560072
- DOI: 10.1016/j.ejrad.2016.12.028
Co-clinical quantitative tumor volume imaging in ALK-rearranged NSCLC treated with crizotinib
Abstract
Purpose: To evaluate and compare the volumetric tumor burden changes during crizotinib therapy in mice and human cohorts with ALK-rearranged non-small-cell lung cancer (NSCLC).
Methods: Volumetric tumor burden was quantified on serial imaging studies in 8 bitransgenic mice with ALK-rearranged adenocarcinoma treated with crizotinib, and in 33 human subjects with ALK-rearranged NSCLC treated with crizotinib. The volumetric tumor burden changes and the time to maximal response were compared between mice and humans.
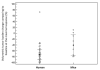
Results: The median tumor volume decrease (%) at the maximal response was -40.4% (range: -79.5%-+11.7%) in mice, and -72.9% (range: -100%-+72%) in humans (Wilcoxon p=0.03). The median time from the initiation of therapy to maximal response was 6 weeks in mice, and 15.7 weeks in humans. Overall volumetric response rate was 50% in mice and 97% in humans. Spider plots of tumor volume changes during therapy demonstrated durable responses in the human cohort, with a median time on therapy of 13.1 months.
Conclusion: The present study described an initial attempt to evaluate quantitative tumor burden changes in co-clinical imaging studies of genomically-matched mice and human cohorts with ALK-rearranged NSCLC treated with crizotinib. Differences are noted in the degree of maximal volume response between the two cohorts in this well-established paradigm of targeted therapy, indicating a need for further studies to optimize co-clinical trial design and interpretation.
Keywords: Anaplastic lymphoma kinase inhibitor; Co-clinical trial; Computed tomography; Non-small-cell lung cancer; Targeted therapy; Tumor volume.
Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.
Figures




References
-
- Ji H, Li D, Chen L, et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell. 2006;9:485–495. - PubMed
-
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

