Fatty-acyl chain profiles of cellular phosphoinositides
- PMID: 28189644
- PMCID: PMC5392126
- DOI: 10.1016/j.bbalip.2017.02.002
Fatty-acyl chain profiles of cellular phosphoinositides
Abstract
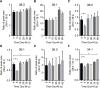
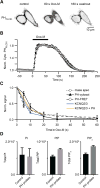
Phosphoinositides are rapidly turning-over phospholipids that play key roles in intracellular signaling and modulation of membrane effectors. Through technical refinements we have improved sensitivity in the analysis of the phosphoinositide PI, PIP, and PIP2 pools from living cells using mass spectrometry. This has permitted further resolution in phosphoinositide lipidomics from cell cultures and small samples of tissue. The technique includes butanol extraction, derivatization of the lipids, post-column infusion of sodium to stabilize formation of sodiated adducts, and electrospray ionization mass spectrometry in multiple reaction monitoring mode, achieving a detection limit of 20pg. We describe the spectrum of fatty-acyl chains in the cellular phosphoinositides. Consistent with previous work in other mammalian primary cells, the 38:4 fatty-acyl chains dominate in the phosphoinositides of the pineal gland and of superior cervical ganglia, and many additional fatty acid combinations are found at low abundance. However, Chinese hamster ovary cells and human embryonic kidney cells (tsA201) in culture have different fatty-acyl chain profiles that change with growth state. Their 38:4 lipids lose their dominance as cultures approach confluence. The method has good time resolution and follows well the depletion in <20s of both PIP2 and PIP that results from strong activation of Gq-coupled receptors. The receptor-activated phospholipase C exhibits no substrate selectivity among the various fatty-acyl chain combinations.
Keywords: Arachidonic acid; Lipidomics; Mass spectrometry; Phospholipids.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures





Similar articles
-
Profiling of Phosphoinositide Molecular Species in Resting or Activated Human or Mouse Platelets by a LC-MS Method.Methods Mol Biol. 2021;2251:39-53. doi: 10.1007/978-1-0716-1142-5_3. Methods Mol Biol. 2021. PMID: 33481230
-
Phosphoinositide profiling in complex lipid mixtures using electrospray ionization mass spectrometry.Nat Biotechnol. 2003 Jul;21(7):813-7. doi: 10.1038/nbt837. Epub 2003 Jun 15. Nat Biotechnol. 2003. PMID: 12808461
-
Phosphate number and acyl chain length determine the subcellular location and lateral mobility of phosphoinositides.Mol Cells. 2006 Aug 31;22(1):97-103. Mol Cells. 2006. PMID: 16951556
-
Mass spectrometry based cellular phosphoinositides profiling and phospholipid analysis: a brief review.Exp Mol Med. 2010 Jan 31;42(1):1-11. doi: 10.3858/emm.2010.42.1.001. Exp Mol Med. 2010. PMID: 19887898 Free PMC article. Review.
-
A new approach to measuring phosphoinositides in cells by mass spectrometry.Adv Biol Regul. 2014 Jan;54:131-41. doi: 10.1016/j.jbior.2013.09.001. Epub 2013 Sep 17. Adv Biol Regul. 2014. PMID: 24120934 Review.
Cited by
-
Identification of key molecular biomarkers involved in reactive and neurodegenerative processes present in inherited congenital hydrocephalus.Fluids Barriers CNS. 2021 Jul 2;18(1):30. doi: 10.1186/s12987-021-00263-2. Fluids Barriers CNS. 2021. PMID: 34215285 Free PMC article.
-
Single-molecule lipid biosensors mitigate inhibition of endogenous effector proteins.J Cell Biol. 2025 Mar 3;224(3):e202412026. doi: 10.1083/jcb.202412026. Epub 2025 Feb 11. J Cell Biol. 2025. PMID: 39932556 Free PMC article.
-
Phosphoinositide Metabolism: Biochemistry, Physiology and Genetic Disorders.J Inherit Metab Dis. 2025 Mar;48(2):e70008. doi: 10.1002/jimd.70008. J Inherit Metab Dis. 2025. PMID: 40024625 Free PMC article. Review.
-
PLCγ1 suppression promotes the adaptation of KRAS-mutant lung adenocarcinomas to hypoxia.Nat Cell Biol. 2020 Nov;22(11):1382-1395. doi: 10.1038/s41556-020-00592-8. Epub 2020 Oct 19. Nat Cell Biol. 2020. PMID: 33077911 Free PMC article.
-
How is the acyl chain composition of phosphoinositides created and does it matter?Biochem Soc Trans. 2019 Oct 31;47(5):1291-1305. doi: 10.1042/BST20190205. Biochem Soc Trans. 2019. PMID: 31657437 Free PMC article. Review.
References
-
- Wenk MR, Lucast L, Di Paolo G, Romanelli AJ, Suchy SF, Nussbaum RL, Cline GW, Shulman GI, McMurray W, De Camilli P. Phosphoinositide profiling in complex lipid mixtures using electrospray ionization mass spectrometry. Nat Biotechnol. 2003;21:813–817. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

