Continuous dosing versus interrupted therapy with ixekizumab: an integrated analysis of two phase 3 trials in psoriasis
- PMID: 28190255
- PMCID: PMC5485049
- DOI: 10.1111/jdv.14163
Continuous dosing versus interrupted therapy with ixekizumab: an integrated analysis of two phase 3 trials in psoriasis
Erratum in
-
Corrigendum.J Eur Acad Dermatol Venereol. 2017 Oct;31(10):1764. doi: 10.1111/jdv.14529. J Eur Acad Dermatol Venereol. 2017. PMID: 29059512 Free PMC article. No abstract available.
Abstract
Background: Continuous treatment is recommended for patients with moderate-to-severe psoriasis; however, treatment may need to be interrupted in routine clinical practice.
Objective: To assess outcomes in patients continuously treated with ixekizumab versus those who interrupted therapy and were subsequently retreated with ixekizumab (IXE).
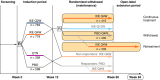
Methods: This analysis used data pooled from two phase 3 trials, UNCOVER-1 and UNCOVER-2. Patients were randomized to placebo (PBO), IXE every 4 (Q4W) or IXE every 2 weeks (Q2W) for 12 weeks. Patients with a static Physician's Global Assessment (sPGA) 0, 1 at Week 12 were rerandomized to IXEQ4W, IXE every 12 weeks (not presented) or PBO. We examined outcomes in patients who were continuously treated (IXEQ2W/IXEQ4W; IXEQ4W/IXEQ4W) or withdrawn (IXEQ2W/PBO; IXEQ4W/PBO), and in patients who were withdrawn and retreated with IXEQ4W for 24 weeks after disease relapse (sPGA ≥3).
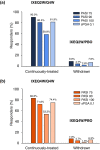
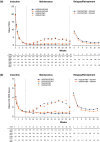
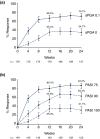
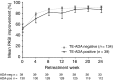
Results: A total of 1226 treated patients achieved an sPGA 0, 1 at Week 12 and entered the maintenance phase; of these patients, 402 and 416 were rerandomized to PBO and IXEQ4W, respectively. Among patients interrupting treatment, 157 (82.2%) of IXEQ4W/PBO and 176 (83.4%) of IXEQ2W/PBO had an sPGA ≥3 by Week 60; median time to relapse was approximately 20 weeks irrespective of induction dose. At Week 60, continuously treated patients maintained high levels of PASI and sPGA responses (90.0% PASI 75 IXEQ2W/IXEQ4W; 81.9% sPGA 0, 1 IXEQ2W/IXEQ4W, non-responder imputation). After 24 weeks of retreatment with IXEQ4W (IXEQ2W/PBO/IXEQ4W and IXEQ4W/PBO/IXEQ4W), 87.0% (107 of 123) and 95.1% (97 of 102) (observed), respectively, of patients recaptured PASI 75 and 70.7% (104 of 147) and 82.3% (107 of 130) (observed) recaptured an sPGA 0, 1. Overall, adverse events in continuously treated and retreated patients were comparable.
Conclusion: High levels of response were sustained with continuous ixekizumab treatment through 60 weeks. Most patients who were withdrawn experienced disease relapse, and most of those patients recaptured response after 24 weeks of retreatment.
© 2017 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Figures
References
-
- Ramirez‐Fort MK, Levin AA, Au SC, Gottlieb AB. Continuous versus intermittent therapy for moderate‐to‐severe psoriasis. Clin Exp Rheumatol 2013; 31(4 Suppl 78): S63–S70. - PubMed
-
- Mrowietz U, de Jong EM, Kragballe K et al A consensus report on appropriate treatment optimization and transitioning in the management of moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol 2014; 28: 438–453. - PubMed
-
- Papp K, Crowley J, Ortonne JP et al Adalimumab for moderate to severe chronic plaque psoriasis: efficacy and safety of retreatment and disease recurrence following withdrawal from therapy. Br J Dermatol 2011; 164: 434–441. - PubMed
-
- Langley RG, Gordon KB. Duration of remission of biologic agents for chronic plaque psoriasis. J Drugs Dermatol 2007; 6: 1205–1212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

