β Cells that Resist Immunological Attack Develop during Progression of Autoimmune Diabetes in NOD Mice
- PMID: 28190773
- PMCID: PMC5342930
- DOI: 10.1016/j.cmet.2017.01.005
β Cells that Resist Immunological Attack Develop during Progression of Autoimmune Diabetes in NOD Mice
Abstract
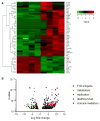
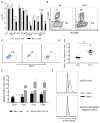
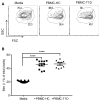
Type 1 diabetes (T1D) is a chronic autoimmune disease that involves immune-mediated destruction of β cells. How β cells respond to immune attack is unknown. We identified a population of β cells during the progression of T1D in non-obese diabetic (NOD) mice that survives immune attack. This population develops from normal β cells confronted with islet infiltrates. Pathways involving cell movement, growth and proliferation, immune responses, and cell death and survival are activated in these cells. There is reduced expression of β cell identity genes and diabetes antigens and increased immune inhibitory markers and stemness genes. This new subpopulation is resistant to killing when diabetes is precipitated with cyclophosphamide. Human β cells show similar changes when cultured with immune cells. These changes may account for the chronicity of the disease and the long-term survival of β cells in some patients.
Keywords: autoimmunity; immune regulation; stem cell; β cell.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Autoimmunity: Death-defying β cells.Nat Rev Endocrinol. 2017 Apr;13(4):189. doi: 10.1038/nrendo.2017.23. Epub 2017 Feb 24. Nat Rev Endocrinol. 2017. PMID: 28232664 No abstract available.
References
-
- Akirav EM, Baquero MT, Opare-Addo LW, Akirav M, Galvan E, Kushner JA, Rimm DL, Herold KC. Glucose and inflammation control islet vascular density and beta-cell function in NOD mice: control of islet vasculature and vascular endothelial growth factor by glucose. Diabetes. 2011;60:876–883. - PMC - PubMed
-
- Bader E, Migliorini A, Gegg M, Moruzzi N, Gerdes J, Roscioni SS, Bakhti M, Brandl E, Irmler M, Beckers J, et al. Identification of proliferative and mature β-cells in the islets of Langerhans. Nature. 2016;535:430–434. - PubMed
-
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

