Reduced platelet hyper-reactivity and platelet-leukocyte aggregation after periodontal therapy
- PMID: 28190975
- PMCID: PMC5292810
- DOI: 10.1186/s12959-016-0125-x
Reduced platelet hyper-reactivity and platelet-leukocyte aggregation after periodontal therapy
Abstract
Background: Platelets from untreated periodontitis patients are hyper-reactive and form more platelet-leukocyte complexes compared to cells from individuals without periodontitis. It is not known whether the improvement of the periodontal condition achievable by therapy has beneficial effects on the platelet function. We aimed to assess the effects of periodontal therapy on platelet reactivity.
Methods: Patients with periodontitis (n = 25) but unaffected by any other medical condition or medication were included and donated blood before and after periodontal therapy. Reactivity to ADP or oral bacteria was assessed by flow cytometric analysis of membrane markers (binding of PAC-1, P-selectin, CD63) and platelet-leukocyte complex formation. Reactivity values were expressed as ratio between the stimulated and unstimulated sample. Plasma levels of soluble (s) P-selectin were determined by enzyme-linked immunosorbent assay (ELISA).
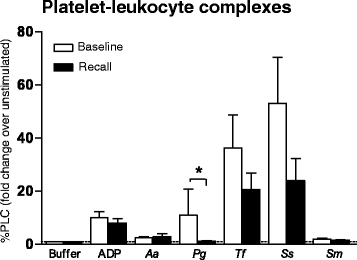
Results: Binding of PAC-1, the expression of P-selectin and CD63 in response to the oral bacterium P. gingivalis were lower at recall (1.4 ± 1.1, 1.5 ± 1.2, and 1.0 ± 0.1) than at baseline (2.7 ± 4.1, P = 0.026, 6.0 ± 12.5, P = 0.045, and 2.7 ± 6.7, P = 0.042, respectively). Formation of platelet-leukocyte complexes in response to P. gingivalis was also reduced at recall compared to baseline (1.2 ± 0.7 vs. 11.4 ± 50.5, P = 0.045). sP-selectin levels were significantly increased post-therapy.
Conclusions: In periodontitis patients, the improvement of the periodontal condition is paralleled by a reduction in platelet hyper-reactivity. We suggest that periodontal therapy, as an intervention for improved oral health, can facilitate the management of thrombotic risk, and on the long term can contribute to the prevention of cardiovascular events in patients at risk.
Trial registration: Current Controlled Trials identifier ISRCTN36043780. Retrospectively registered 25 September 2013.
Keywords: Periodontal treatment; Periodontitis; Platelet reactivity; Platelet-monocyte complexes; Platelet-neutrophil Complexes.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

