From egg to "no-body": an overview and revision of developmental pathways in the ancient arthropod lineage Pycnogonida
- PMID: 28191025
- PMCID: PMC5297176
- DOI: 10.1186/s12983-017-0192-2
From egg to "no-body": an overview and revision of developmental pathways in the ancient arthropod lineage Pycnogonida
Abstract
Background: Arthropod diversity is unparalleled in the animal kingdom. The study of ontogeny is pivotal to understand which developmental processes underlie the incredible morphological disparity of arthropods and thus to eventually unravel evolutionary transformations leading to their success. Work on laboratory model organisms has yielded in-depth data on numerous developmental mechanisms in arthropods. Yet, although the range of studied taxa has increased noticeably since the advent of comparative evolutionary developmental biology (evo-devo), several smaller groups remain understudied. This includes the bizarre Pycnogonida (sea spiders) or "no-bodies", a taxon occupying a crucial phylogenetic position for the interpretation of arthropod development and evolution.
Results: Pycnogonid development is variable at familial and generic levels and sometimes even congeneric species exhibit different developmental modes. Here, we summarize the available data since the late 19th century. We clarify and resolve terminological issues persisting in the pycnogonid literature and distinguish five developmental pathways, based on (1) type of the hatching stage, (2) developmental-morphological features during postembryonic development and (3) selected life history characteristics. Based on phylogenetic analyses and the fossil record, we discuss plausible plesiomorphic features of pycnogonid development that allow comparison to other arthropods. These features include (1) a holoblastic, irregular cleavage with equal-sized blastomeres, (2) initiation of gastrulation by a single bottle-shaped cell, (3) the lack of a morphologically distinct germ band during embryogenesis, (4) a parasitic free-living protonymphon larva as hatching stage and (5) a hemianamorphic development during the postlarval and juvenile phases. Further, we propose evolutionary developmental trajectories within crown-group Pycnogonida.
Conclusions: A resurgence of studies on pycnogonid postembryonic development has provided various new insights in the last decades. However, the scarcity of modern-day embryonic data - including the virtual lack of gene expression and functional studies - needs to be addressed in future investigations to strengthen comparisons to other arthropods and arthropod outgroups in the framework of evo-devo. Our review may serve as a basis for an informed choice of target species for such studies, which will not only shed light on chelicerate development and evolution but furthermore hold the potential to contribute important insights into the anamorphic development of the arthropod ancestor.
Keywords: Anamorphic development; Arthropoda; Embryology; Evo-devo; Evolution; Gastrulation; Postembryonic development; Protonymphon larva; Sea spider.
Figures
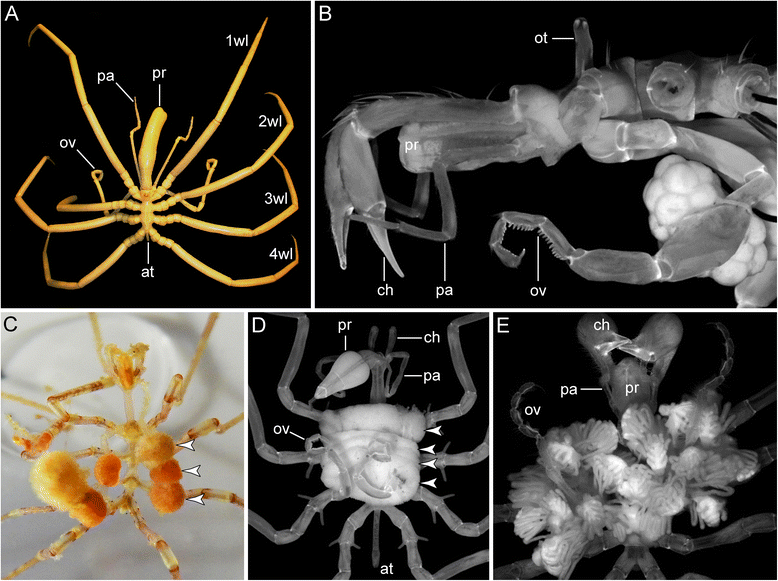
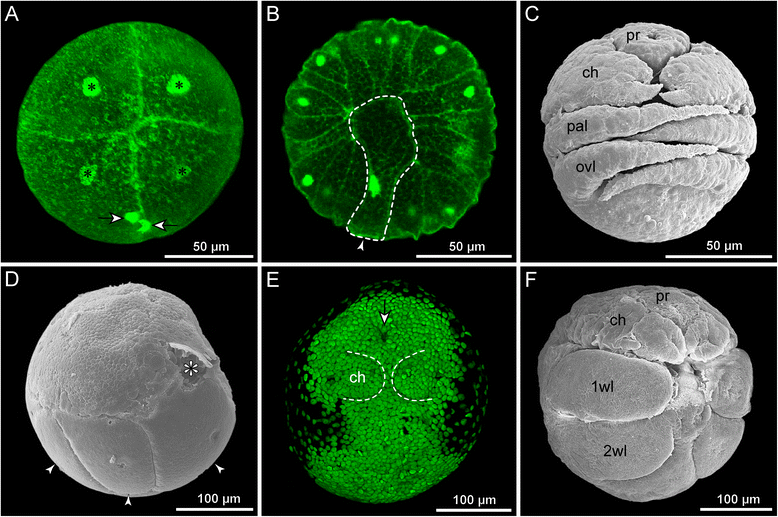

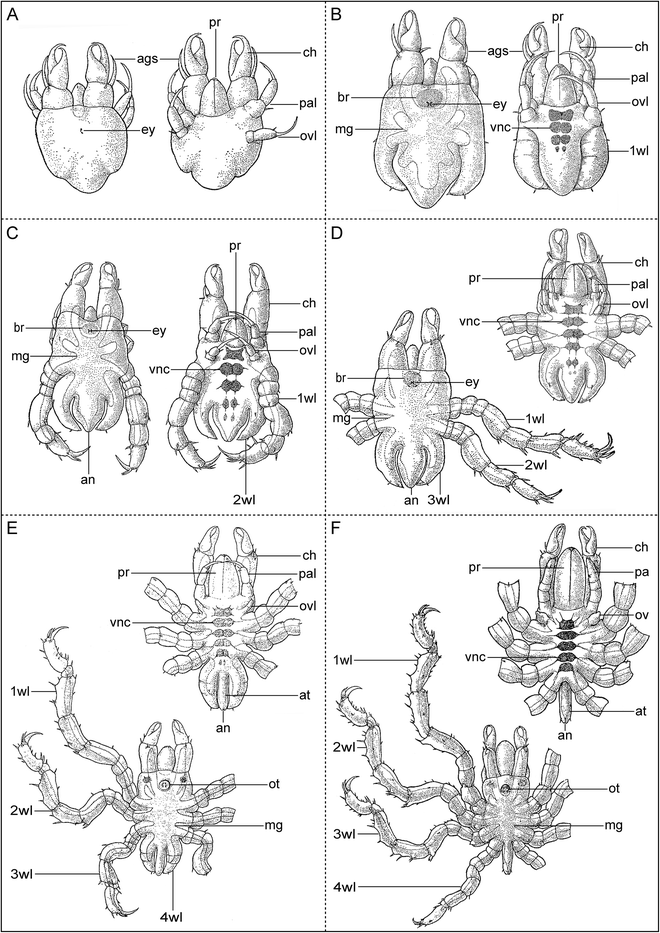
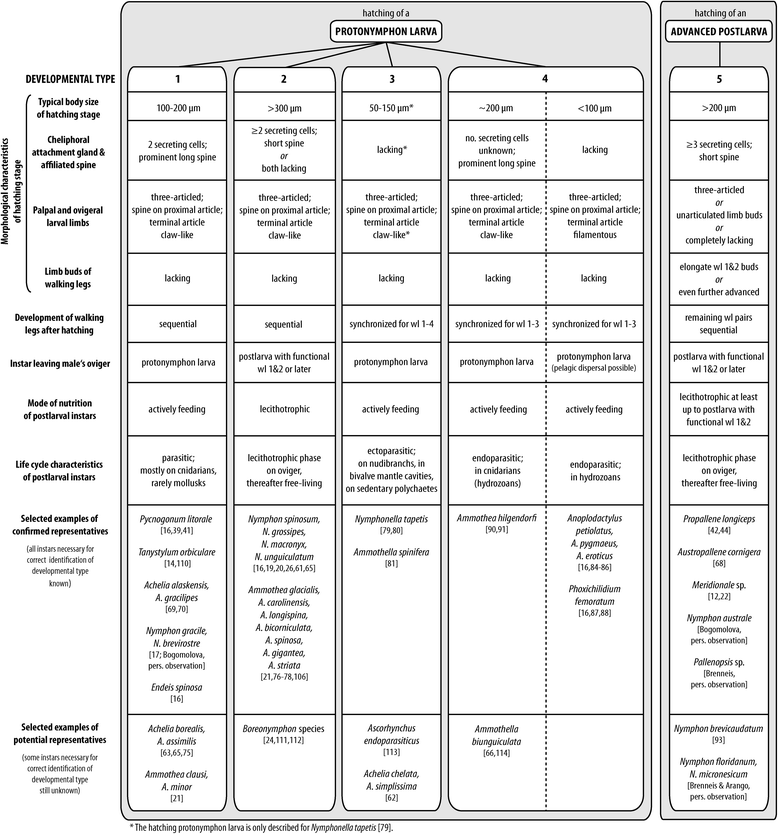


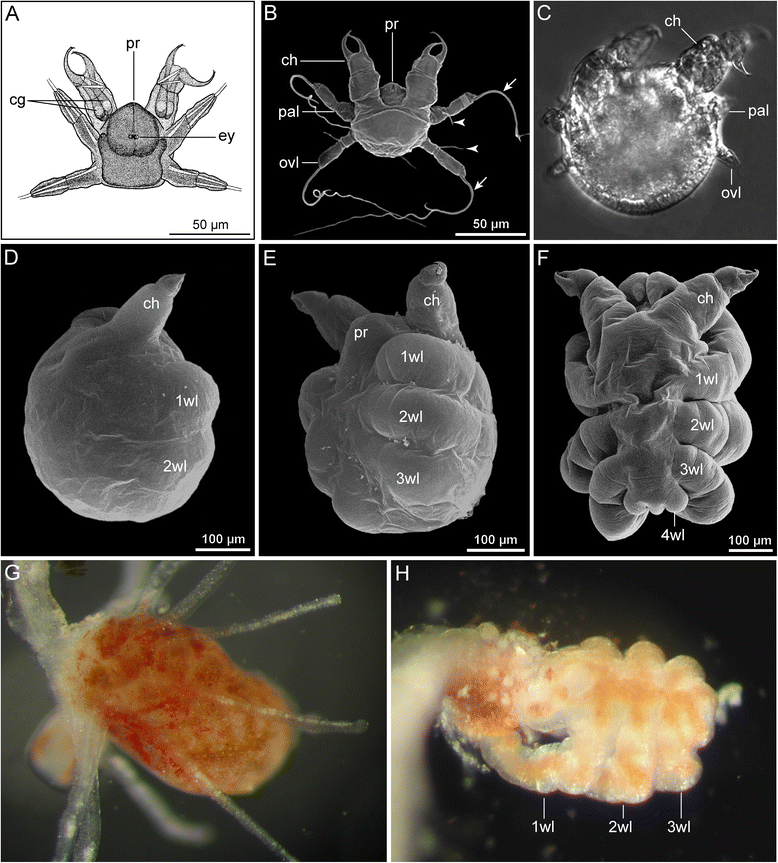
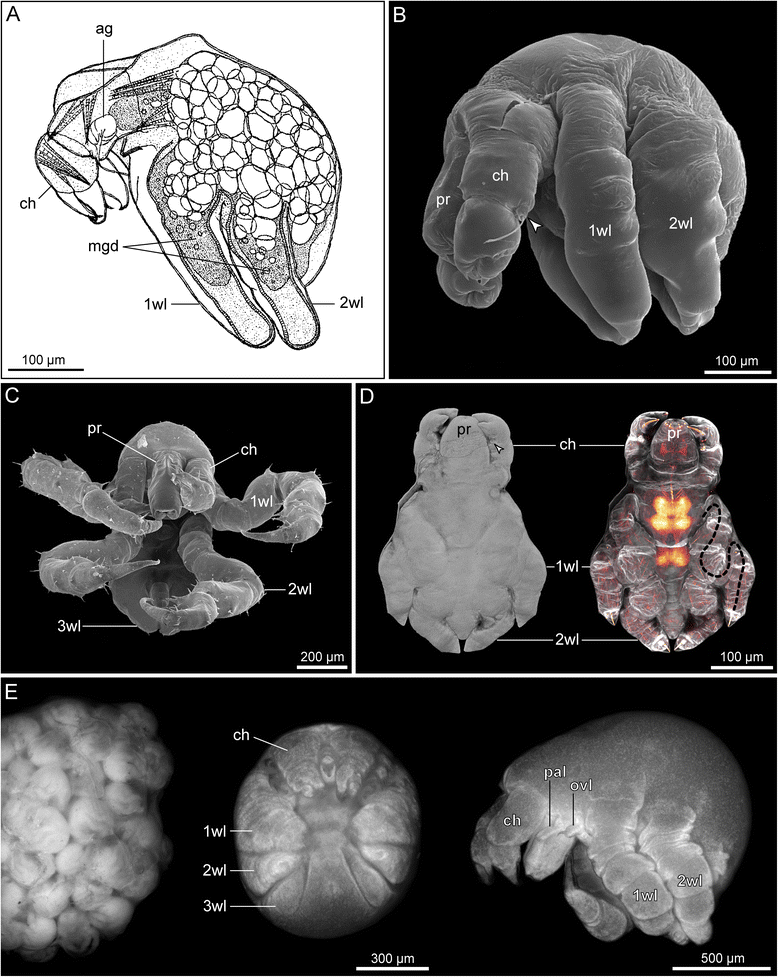
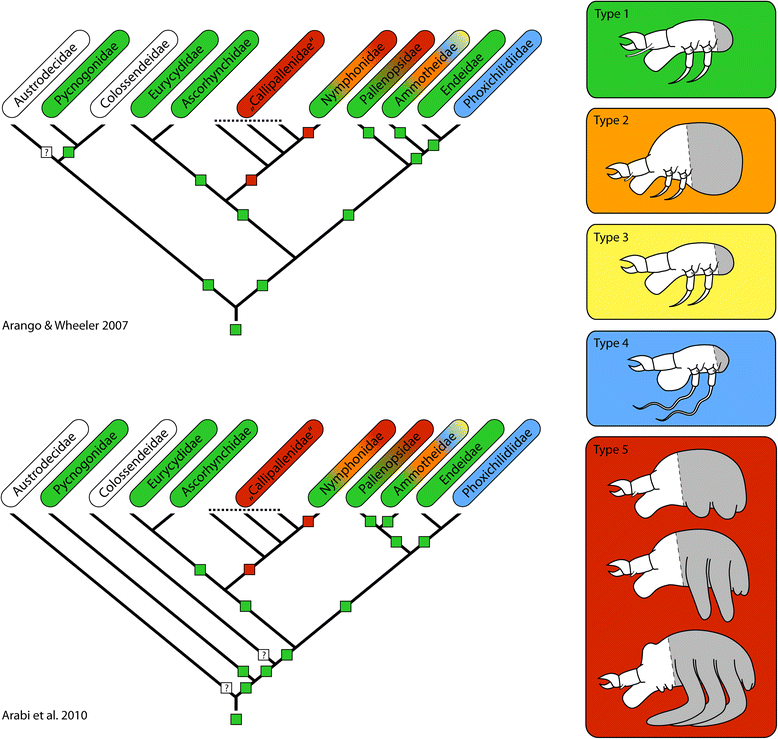
References
-
- Giribet G, Edgecombe GD. The Arthropoda: A Phylogenetic Framework. In: Minelli A, Boxshall G, Fusco G, editors. Arthropod Biology and Evolution Molecules, Development, Morphology. Heidelberg: Springer Verlag; 2013. pp. 17–40.
-
- Richter S, Wirkner C. A research program for Evolutionary Morphology. J Zool Syst Evol Res. 2014;52:338–350. doi: 10.1111/jzs.12061. - DOI
-
- Dunlop JA, Arango CP. Pycnogonid affinities: a review. J Zool Syst Evol Res. 2005;43:8–21. doi: 10.1111/j.1439-0469.2004.00284.x. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

