The role of epicardial adipose tissue in cardiac biology: classic concepts and emerging roles
- PMID: 28191635
- PMCID: PMC5471417
- DOI: 10.1113/JP273049
The role of epicardial adipose tissue in cardiac biology: classic concepts and emerging roles
Abstract
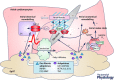
Classic concepts about the role of epicardial adipose tissue (EpAT) in heart physiology include its role in cardiac metabolism, mechanical protection of coronaries, innervation and possibly cryoprotection of the heart too. Nevertheless, recent evidence has revealed that epicardial adipose tissue regulates multiple aspects of cardiac biology including myocardial redox state, intracellular Ca2+ cycling, the electrophysiological and contractile properties of cardiomyocytes, cardiac fibrosis as well as coronary atherosclerosis progression. Moreover, it is now understood that the communication between EpAT and the heart is regulated by complex bidirectional pathways, since not only do adipokines regulate cardiac function, but also the heart affects EpAT biology via paracrine 'reverse' signalling. Such complex interactions as well as epicardial fat accumulation as a consequence of cardiac disease and epicardium to adipocyte differentiation should be taken into account by the clinical studies investigating EpAT as a risk marker and its potential as a therapeutic target against cardiovascular disease. Further in-depth exploration of the molecular mechanisms regulating the cross-talk between the heart and EpAT is expected to enhance our understanding regarding the role of the latter in cardiac physiology and relevant disease mechanisms.
Keywords: adipokines; cardiac biology; epicardial adipose tissue; myocardium; redox state.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures



References
-
- Anan M, Uchihashi K, Aoki S, Matsunobu A, Ootani A, Node K & Toda S (2011). A promising culture model for analyzing the interaction between adipose tissue and cardiomyocytes. Endocrinology 152, 1599–1605. - PubMed
-
- Antoniades C, Demosthenous M, Reilly S, Margaritis M, Zhang MH, Antonopoulos A, Marinou K, Nahar K, Jayaram R, Tousoulis D, Bakogiannis C, Sayeed R, Triantafyllou C, Koumallos N, Psarros C, Miliou A, Stefanadis C, Channon KM & Casadei B (2012). Myocardial redox state predicts in‐hospital clinical outcome after cardiac surgery effects of short‐term pre‐operative statin treatment. J Am Coll Cardiol 59, 60–70. - PubMed
-
- Antonopoulos AS, Margaritis M, Coutinho P, Digby J, Patel R, Psarros C, Ntusi N, Karamitsos TD, Lee R, De Silva R, Petrou M, Sayeed R, Demosthenous M, Bakogiannis C, Wordsworth PB, Tousoulis D, Neubauer S, Channon KM & Antoniades C (2014). Reciprocal effects of systemic inflammation and brain natriuretic peptide on adiponectin biosynthesis in adipose tissue of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol 34, 2151–2159. - PubMed
-
- Antonopoulos AS, Margaritis M, Coutinho P, Shirodaria C, Psarros C, Herdman L, Sanna F, De Silva R, Petrou M, Sayeed R, Krasopoulos G, Lee R, Digby J, Reilly S, Bakogiannis C, Tousoulis D, Kessler B, Casadei B, Channon KM & Antoniades C (2015). Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Diabetes 64, 2207–2219. - PubMed
-
- Antonopoulos AS, Margaritis M, Verheule S, Recalde A, Sanna F, Herdman L, Psarros C, Nasrallah H, Coutinho P, Akoumianakis I, Brewer AC, Sayeed R, Krasopoulos G, Petrou M, Tarun A, Tousoulis D, Shah AM, Casadei B, Channon KM & Antoniades C (2016a). Mutual regulation of epicardial adipose tissue and myocardial redox state by PPAR‐γ/adiponectin signalling. Circ Res 118, 842–855. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

