Directing Differentiation of Pluripotent Stem Cells Toward Retinal Pigment Epithelium Lineage
- PMID: 28191760
- PMCID: PMC5442825
- DOI: 10.5966/sctm.2016-0088
Directing Differentiation of Pluripotent Stem Cells Toward Retinal Pigment Epithelium Lineage
Abstract
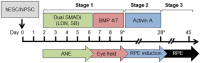
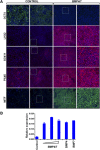
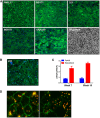
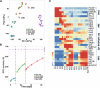
Development of efficient and reproducible conditions for directed differentiation of pluripotent stem cells into specific cell types is important not only to understand early human development but also to enable more practical applications, such as in vitro disease modeling, drug discovery, and cell therapies. The differentiation of stem cells to retinal pigment epithelium (RPE) in particular holds promise as a source of cells for therapeutic replacement in age-related macular degeneration. Here we show development of an efficient method for deriving homogeneous RPE populations in a period of 45 days using an adherent, monolayer system and defined xeno-free media and matrices. The method utilizes sequential inhibition and activation of the Activin and bone morphogenetic protein signaling pathways and can be applied to both human embryonic stem cells and induced pluripotent stem cells as the starting population. In addition, we use whole genome transcript analysis to characterize cells at different stages of differentiation that provides further understanding of the developmental dynamics and fate specification of RPE. We show that with the described method, RPE develop through stages consistent with their formation during embryonic development. This characterization- together with the absence of steps involving embryoid bodies, three-dimensional culture, or manual dissections, which are common features of other protocols-makes this process very attractive for use in research as well as for clinical applications. Stem Cells Translational Medicine 2017;6:490-501.
Keywords: Activin; Bone morphogenetic protein; Directed differentiation; Retinal pigment epithelium; Stem cells.
© 2016 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures

References
-
- Friedman DS, O'Colmain BJ, Muñoz B et al. Prevalence of age‐related macular degeneration in the United States. Arch Ophthalmol 2004;122:564–572. - PubMed
-
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev 2005;85:845–881. - PubMed
-
- Schwartz SD, Hubschman JP, Heilwell G et al. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet 2012;379:713–720. - PubMed
-
- Schwartz SD, Regillo CD, Lam BL et al. Human embryonic stem cell‐derived retinal pigment epithelium in patients with age‐related macular degeneration and Stargardt's macular dystrophy: Follow‐up of two open‐label phase 1/2 studies. Lancet 2015;385:509–516. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

