Angiogenic Potential of Human Bone Marrow-Derived Mesenchymal Stem Cells in Chondrocyte Brick-Enriched Constructs Promoted Stable Regeneration of Craniofacial Cartilage
- PMID: 28191761
- PMCID: PMC5442805
- DOI: 10.5966/sctm.2016-0050
Angiogenic Potential of Human Bone Marrow-Derived Mesenchymal Stem Cells in Chondrocyte Brick-Enriched Constructs Promoted Stable Regeneration of Craniofacial Cartilage
Abstract
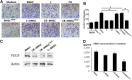
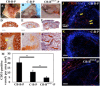
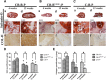
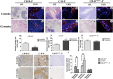
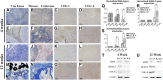
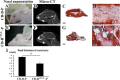
Craniofacial deformities caused by congenital defects or trauma remain challenges for clinicians, whereas current surgical interventions present limited therapeutic outcomes. Injection of bone marrow-derived mesenchymal stem cells (BMSCs) into the defect is highly desirable because such a procedure is microinvasive and grafts are more flexible to fill the lesions. However, preventing hypertrophic transition and morphological contraction remain significant challenges. We have developed an "all host derived" cell transplantation system composed of chondrocyte brick (CB)-enriched platelet-rich plasma (P) gel and BMSCs (B). Without exogenous biomaterials or growth factors, such grafts regenerate cartilage efficiently and present great clinical promise. In immunodeficient mice, we compared performance of BMSCs and BMSCs lacking angiogenic potential in CB-B-P constructs and followed the cartilage maturation process by histology, immunostaining, micro-computed tomography, and protein analysis. We determined that angiogenesis occurred quickly inside rudimentary cartilage derived from CB-B-P constructs after implantation, which improved tissue survival, tissue growth, and production of chondrogenic signals from chondrocytes. In contrast, silencing angiogenic potential of BMSCs led to poor chondrogenesis accompanied by necrosis. Chondrocyte bricks merged rapidly with angiogenesis, which constituted an enclosed chondrogenic niche and effectively inhibited runt-related transcription factor-2-dependent hypertrophic transition of BMSCs as well as endochondral ossification; progressive chondrogenic differentiation of BMSCs resulted in vascularization regression, thus favoring persistent chondrogenesis and effectively augmenting nasal cartilage. In conclusion, these findings provided a novel, efficient approach to regenerating cartilage tissues in vivo. Chondrocyte bricks mixed with P provide transient vascularization and a persistently chondrogenic microenvironment for BMSCs; this provides a mini-invasive approach for craniofacial cartilage reconstruction. Stem Cells Translational Medicine 2017;6:601-612.
Keywords: Adult human bone marrow; Angiogenesis; Bone marrow stromal cells; Cell transplantation; Chondrogenesis.
© 2016 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures

References
-
- Fulco I, Miot S, Haug MD et al. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: An observational first‐in‐human trial. Lancet 2014;384:337–346. - PubMed
-
- Sasai Y. Next‐generation regenerative medicine: Organogenesis from stem cells in 3D culture. Cell Stem Cell 2013;12:520–530. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

