Preventing Pluripotent Cell Teratoma in Regenerative Medicine Applied to Hematology Disorders
- PMID: 28191782
- PMCID: PMC5442801
- DOI: 10.5966/sctm.2016-0201
Preventing Pluripotent Cell Teratoma in Regenerative Medicine Applied to Hematology Disorders
Abstract
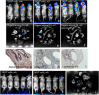
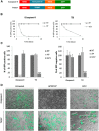
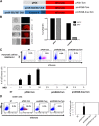
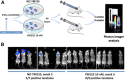
Iatrogenic tumorigenesis is a major limitation for the use of human induced pluripotent stem cells (hiPSCs) in hematology. The teratoma risk comes from the persistence of hiPSCs in differentiated cell populations. Our goal was to evaluate the best system to purge residual hiPSCs before graft without compromising hematopoietic repopulation capability. Teratoma risk after systemic injection of hiPSCs expressing the reporter gene luciferase was assessed for the first time. Teratoma formation in immune-deficient mice was tracked by in vivo bioimaging. We observed that systemic injection of hiPSCs produced multisite teratoma as soon as 5 weeks after injection. To eliminate hiPSCs before grafting, we tested the embryonic-specific expression of suicide genes under the control of the pmiR-302/367 promoter. This promoter was highly active in hiPSCs but not in differentiated cells. The gene/prodrug inducible Caspase-9 (iCaspase-9)/AP20187 was more efficient and rapid than thymidine kinase/ganciclovir, fully specific, and without bystander effect. We observed that iCaspase-9-expressing hiPSCs died in a dose-dependent manner with AP20187, without reaching full eradication in vitro. Unexpectedly, nonspecific toxicity of AP20187 on iCaspase-9-negative hiPSCs and on CD34+ cells was evidenced in vitro. This toxic effect strongly impaired CD34+ -derived human hematopoiesis in adoptive transfers. Survivin inhibition is an alternative to the suicide gene approach because hiPSCs fully rely on survivin for survival. Survivin inhibitor YM155 was more efficient than AP20187/iCaspase-9 for killing hiPSCs, without toxicity on CD34+ cells, in vitro and in adoptive transfers. hiPSC purge by survivin inhibitor fully eradicated teratoma formation in immune-deficient mice. This will be useful to improve the safety management for hiPSC-based medicine. Stem Cells Translational Medicine 2017;6:382-393.
Keywords: Hematology; Hematopoietic stem cell; Induced pluripotent stem cells; Regenerative medicine; Safety; Suicide gene; Survivin inhibitor; Teratoma; Thymidine kinase; iCaspase-9.
© 2016 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

