POMC Neurons: From Birth to Death
- PMID: 28192062
- PMCID: PMC5669621
- DOI: 10.1146/annurev-physiol-022516-034110
POMC Neurons: From Birth to Death
Abstract
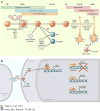
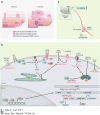
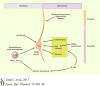
The hypothalamus is an evolutionarily conserved brain structure that regulates an organism's basic functions, such as homeostasis and reproduction. Several hypothalamic nuclei and neuronal circuits have been the focus of many studies seeking to understand their role in regulating these basic functions. Within the hypothalamic neuronal populations, the arcuate melanocortin system plays a major role in controlling homeostatic functions. The arcuate pro-opiomelanocortin (POMC) neurons in particular have been shown to be critical regulators of metabolism and reproduction because of their projections to several brain areas both in and outside of the hypothalamus, such as autonomic regions of the brain stem and spinal cord. Here, we review and discuss the current understanding of POMC neurons from their development and intracellular regulators to their physiological functions and pathological dysregulation.
Keywords: POMC; development; food intake; hypothalamus; obesity.
Figures
References
-
- Horvath TL, Naftolin F, Kalra SP, Leranth C. Neuropeptide-Y innervation of beta-endorphin-containing cells in the rat mediobasal hypothalamus: a light and electron microscopic double immunostaining analysis. Endocrinology. 1992;131:2461–67. - PubMed
-
- Sternson SM, Atasoy D, Betley JN, Henry FE, Xu S. An emerging technology framework for the neurobiology of appetite. Cell Metab. 2016;23:234–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

