Retinal origin of direction selectivity in the superior colliculus
- PMID: 28192394
- PMCID: PMC5374021
- DOI: 10.1038/nn.4498
Retinal origin of direction selectivity in the superior colliculus
Abstract
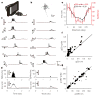
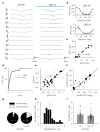
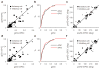
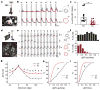
Detecting visual features in the environment, such as motion direction, is crucial for survival. The circuit mechanisms that give rise to direction selectivity in a major visual center, the superior colliculus (SC), are entirely unknown. We optogenetically isolate the retinal inputs that individual direction-selective SC neurons receive and find that they are already selective as a result of precisely converging inputs from similarly tuned retinal ganglion cells. The direction-selective retinal input is linearly amplified by intracollicular circuits without changing its preferred direction or level of selectivity. Finally, using two-photon calcium imaging, we show that SC direction selectivity is dramatically reduced in transgenic mice that have decreased retinal selectivity. Together, our studies demonstrate a retinal origin of direction selectivity in the SC and reveal a central visual deficit as a consequence of altered feature selectivity in the retina.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Cang J, Feldheim DA. Developmental mechanisms of topographic map formation and alignment. Annu Rev Neurosci. 2013;36:51–77. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

