Defining the antibody cross-reactome directed against the influenza virus surface glycoproteins
- PMID: 28192418
- PMCID: PMC5360498
- DOI: 10.1038/ni.3684
Defining the antibody cross-reactome directed against the influenza virus surface glycoproteins
Abstract
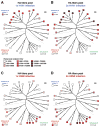
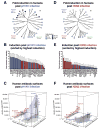
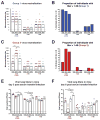
Infection with influenza virus induces antibodies to the viral surface glycoproteins hemagglutinin and neuraminidase, and these responses can be broadly protective. To assess the breadth and magnitude of antibody responses, we sequentially infected mice, guinea pigs and ferrets with divergent H1N1 or H3N2 subtypes of influenza virus. We measured antibody responses by ELISA of an extensive panel of recombinant glycoproteins representing the viral diversity in nature. Guinea pigs developed high titers of broadly cross-reactive antibodies; mice and ferrets exhibited narrower humoral responses. Then, we compared antibody responses after infection of humans with influenza virus H1N1 or H3N2 and found markedly broad responses and cogent evidence for 'original antigenic sin'. This work will inform the design of universal vaccines against influenza virus and can guide pandemic-preparedness efforts directed against emerging influenza viruses.
Conflict of interest statement
The authors have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

