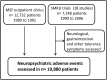
Adverse effects of mefloquine for the treatment of uncomplicated malaria in Thailand: A pooled analysis of 19, 850 individual patients
- PMID: 28192434
- PMCID: PMC5305067
- DOI: 10.1371/journal.pone.0168780
Adverse effects of mefloquine for the treatment of uncomplicated malaria in Thailand: A pooled analysis of 19, 850 individual patients
Abstract
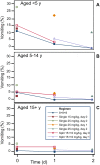
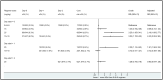
Mefloquine (MQ) has been used for the treatment of malaria since the mid-1980s, first as monotherapy or as fixed combination with sulfadoxine-pyrimethamine (MSP) and since the mid-1990s in combination with artesunate. There is a renewed interested in MQ as part of a triple therapy for the treatment of multi-drug resistance P. falciparum malaria. The widespread use of MQ beyond south-East Asia has been constrained by reports of poor tolerability. Here we present the side effect profile of MQ for the treatment of uncomplicated malaria on the Thai-Myanmar/Cambodia borders. In total 19,850 patients received seven different regimens containing either 15 or 24-25 mg/kg of MQ, the latter given either as a single dose, or split over two or three days. The analysis focused on (predominantly) gastrointestinal and neuropsychiatric events as compared to the new fixed dose combination of MQ plus artesunate given as equal doses of 8 mg/kg MQ per day over three days. Gastrointestinal side effects were dose-dependent and associated with the severity of malaria symptoms. Serious neuropsychiatric side effects associated with MQ use were rare: for a single 25 mg/kg dose it was 11.9 per 10,000 treatments (95% confidence interval, CI, 4-285) vs. 7.8 (3-15) for the 15 mg/kg dose. The risk with 25 mg/kg was much higher when it was given as repeat dosing in patients who had failed treatment with 15 mg/kg MQ in the preceding month; (RR 6.57 (95% CI 1.33 to 32.4), p = 0.0077). MQ was best tolerated as 15 mg/kg or as 24 mg/kg when given over three days in combination with artesunate. We conclude that the tolerance of a single dose of MQ in the treatment of uncomplicated malaria is moderate, but can be improved by administering it as a split dose over three days.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

