Drug versus placebo randomized controlled trials in neonates: A review of ClinicalTrials.gov registry
- PMID: 28192509
- PMCID: PMC5305102
- DOI: 10.1371/journal.pone.0171760
Drug versus placebo randomized controlled trials in neonates: A review of ClinicalTrials.gov registry
Abstract
Background: Despite specific initiatives and identified needs, most neonatal drugs are still used off-label, with variable dosage administrations and schedules. In high risk preterm and term neonates, drug evaluation is challenging and randomized controlled trials (RCT) are difficult to conduct and even more is the use of a placebo, required in the absence of a reference validated drug to be used as comparator.
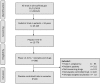
Methods: We analyzed the complete ClinicalTrials.gov registry 1) to describe neonatal RCT involving a placebo, 2) to report on the medical context and ethical aspects of placebo use.
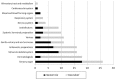
Results: Placebo versus drug RCT (n = 146), either prevention trials (n = 57, 39%) or therapeutic interventions (n = 89, 61%), represent more than a third of neonatal trials registered in the National Institute of Health clinical trial database (USA) since 1999. They mainly concerned preterm infants, evaluating complications of prematurity. Most trials were conducted in the USA, were single centered, and funded by non-profit organizations. For the three top drug trials evaluating steroids (n = 13, 9.6%), erythropoietin (EPO, n = 10, 6.8%) and nitric oxide (NO, n = 9, 6.2%), the objectives of the trial and follow-up were analyzed in more details.
Conclusion: Although a matter of debate, the use of placebo should be promoted in neonates to evaluate a potential new treatment, in the absence of reference drug. Analysis of the trials evaluating steroids showed that long-term follow-up of exposed patients, although required by international guidelines, is frequently missing and should be planned to collect additional information and optimize drug evaluation in these high-risk patients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Jacqz-Aigrain E. Drug policy in Europe Research and funding in neonates: current challenges, future perspectives, new opportunities. Early Hum Dev. 2011;87 Suppl 1: S27–30. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

